The New Food Safety
A safe food supply is essential for a healthy society. Our food system is replete with different types of risk, yet food safety is often narrowly understood as encompassing only foodborne illness and other risks related directly to food ingestion. This Article argues for a more comprehensive definition of food safety, one that includes not just acute, ingestion-related risks, but also whole-diet cumulative ingestion risks, and cradle-to-grave risks of food production and disposal. This broader definition, which we call “Food System Safety,” draws under the header of food safety a variety of historically siloed, and under-regulated, food system issues including nutrition, environmental protection, and workplace safety. The current narrow approach to food safety is inadequate. First, it contributes to irrational resource allocation among food system risks. Second, it has collateral consequences for other food system risks, and, third, its limited focus can undermine efforts to achieve narrow food safety. A comprehensive understanding of food safety illuminates the complex interactions between narrow food safety and other areas of food system health risks. We argue that such an understanding could facilitate improved allocation of resources and assessment of tradeoffs, and ultimately support better health and safety outcomes for more people. We offer a variety of structural and institutional mechanisms for embedding this approach into federal agency action.
Table of Contents Show
Introduction
A child contracts Salmonella poisoning after eating a hamburger. A man is diagnosed with diabetes after several decades of sugar overconsumption. A resident of a community with an animal feedlot has an asthma attack exacerbated by particulate air pollutants from the feedlot. Which of these three people got sick because food is unsafe? There is a strong democratic consensus that a safe food supply is essential for the maintenance of a healthy and prosperous society. But what does “food safety” mean? And how does that definition shape food policy?
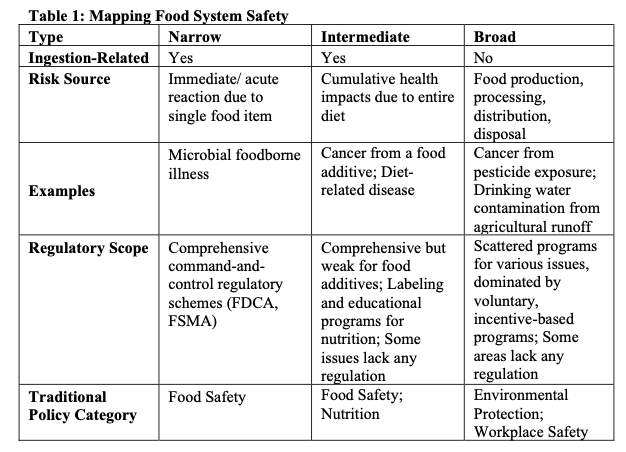
In the United States, “food safety” is often understood as encompassing only foodborne illness. Our food system, however, is susceptible to a broad range of dangers, suggesting that “food safety” could be defined in a variety of ways. In this Article, we posit three theoretical food safety categories. First, narrow food safety refers to acute ingestion-related illness such as microbial contamination from consumption of a single food item. Second, intermediate food safety refers to whole-diet, cumulative ingestion-related risks that accrue over time, such as diabetes or cancer. Finally, broad food safety includes risks that arise from food production or disposal, the impacts of which are felt before and after the point of ingestion. In this Article, we map these three categories, which together we call “Food System Safety,” onto the existing structures of food regulation, and we demonstrate how adopting a food safety definition encompassing all three categories could improve health outcomes.
US food regulation addresses the three categories of food safety under the distinct policy siloes of food safety, nutrition, environmental protection, and workplace safety. The traditional food safety regulatory framework addresses narrow food safety, concerns related to foodborne illness and acute toxicity, and incorporates some elements of intermediate food safety, particularly those related to carcinogenic food additives. Traditional food safety in general and narrow food safety in particular dominate the regulatory regime. Although fifteen different agencies have some regulatory authority in this area, the two most important are the Food and Drug Administration (FDA) and the Department of Agriculture (USDA), which both use a variety of prescriptive regulatory tools to protect consumers from contaminated foods.[2] This Article focuses primarily on the FDA, the agency responsible for the safety of the largest share of the food supply.[3]
Nutrition law covers the remainder of intermediate food safety. The FDA and the USDA address nutrition primarily through education, labeling, and voluntary incentive programs. Finally, to the extent broad food safety is regulated, it is addressed through environmental protection and workplace safety. The Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA) regulate some aspects of workers’ safety in food production, particularly those related to pesticide use. The USDA also regulates the environmental aspects of food production through voluntary incentive-based programs. Many aspects of broad food safety are unregulated or under-regulated.
This Article argues that drawing these issues together into “Food System Safety” will result in a safer food system. Currently, even where risks in the intermediate and broad food safety categories are more severe and more widespread, narrow food safety receives greater funding and more aggressive regulatory measures. A Food System Safety approach will facilitate better resource allocation and regulatory decisions. We also argue that by prioritizing narrow food safety, current regulatory approaches both undermine policy goals in other arenas, and, in some cases, undercut narrow food safety itself by failing to appreciate the complex causal relationships among the various types of food system health risks. A unified approach to food safety could better illuminate the interconnections between these historically distinct issues, which all relate to the same food system activities.
Food System Safety offers a paradigmatic case for evaluating risk management in modern society: it is a complex problem, with nebulous causal chains, paltry and imprecise data, and inequitably distributed benefits and burdens.[4] In this sense, regulating food is no different than regulating any other area of consumer protection. We ask the same core questions here as we would in any area of health and safety regulation: how do we equitably maximize benefits from limited regulatory resources? How do we account for the unintended consequences of regulatory choices? How do we ensure that our regulatory strategies are not so narrowly conceived that they are counterproductive?
So why focus on food? First, food regulation as a whole is undertheorized, and the problems described in this Article have significant consequences.[5] Current food regulation is not as efficient or as effective as it could be.
Second, food safety regulation offers a particularly stark example of the problems associated with myopic risk management.[6] Dividing food system health risks into isolated regulatory categories makes it more difficult for regulators to understand how risks interact with each other and exacerbates the challenges of implementing rational health and safety regulation.[7] Even where regulators do attempt to assess tradeoffs, they typically begin with a primary policy priority, usually narrow food safety, and then examine other policy concerns in light of that goal.[8] How we define “food safety” thus establishes the scope of regulatory missions.[9] A limited definition intensifies agency “tunnel vision,” giving regulators permission to prioritize narrow food safety over other food system health risks and, sometimes, to ignore or shortchange those other risks in the very decision-making processes designed to foster incorporation of broader considerations.[10]
We argue that the urgent need to address our most prevalent and costly food-related health concerns, such as diabetes, heart disease, antibiotic resistance, and air and water pollution, merits a more expansive definition of food safety, what we call “Food System Safety.” This Article maps the relationships between traditional areas of food system health—food safety, nutrition, environmental protection, and workplace safety—and reframes all of these areas as aspects of Food System Safety.
Part I of this Article begins with a description of the full range of food system health risks. It classifies them into three categories: (1) narrow; (2) intermediate; and (3) broad, and it describes current regulatory approaches to each. This discussion demonstrates that narrow food safety receives more robust and comprehensive regulatory treatment than other food system health risks. Part I then draws on history, politics, and economics to understand why narrow food safety dominates.
Part II illustrates the consequences of this constrained regulatory focus. Part II argues that resource allocation does not match risk severity, that prioritizing narrow food safety results in undesirable tradeoffs with nutrition, environmental protection, and workplace safety, and that designing solutions to narrow food safety that do not take a full systems view can, ironically, undermine narrow food safety itself.
Finally, Part III presents proposals for reform that could more strategically deploy resources to reduce food system health risks. It begins by arguing for the importance of changing the definition of food safety to encompass the broader set of food system risks described throughout, and then offers suggestions for structural improvements to the food safety regulatory regime in order to better address these risks.[11]
I. Defining “Food Safety”
Despite the broad range of food system health risks, regulators, lawmakers, and advocates consistently ascribe only a few of these risks to the category of food safety. Food safety typically includes microbial contamination, chemical poisoning, and certain health risks associated with food additives (in particular carcinogenicity). Even advocates for more robust federal protections for food-related health problems accept the current definition of food safety. For instance, food scholar Marion Nestle identifies food safety and nutrition as distinct food system issues, noting that “[i]n recent years, as consumer concerns about diet-related chronic diseases have increased, food laws have increasingly addressed issues of health beyond food safety.”[12] Food safety, narrowly defined, dominates federal food system health regulation. This Part begins with our taxonomy of Food System Safety. We describe how each type of food safety–narrow, intermediate, and broad–is regulated and argue that significant regulatory gaps exist in the latter two categories. The second half of this Part considers a range of explanations for the dominance of narrow food safety.
A. Categories of Food Safety
The FDA characterizes its mission as “protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices; and by ensuring the safety of our nation’s food supply, cosmetics, and products that emit radiation.”[13] Safety, according to Merriam-Webster, is “the condition of being safe from undergoing or causing hurt, injury, or loss.”[14] The Food Drug and Cosmetic Act (FDCA), the primary law governing FDA regulatory authority, defines “safe” by “reference to the health of man or animal.”[15] This definition provides no content to the FDA’s food safety mandate. Instead, that content comes from other substantive statutory directives and agency decisions.
By identifying the range of risks to the “health of man or animal,” we demonstrate the range of possible meanings of “food safety.” First, we label all of the food safety issues related to acute ingestion-related harm as narrow food safety. Next, intermediate food safety encompasses both traditional food safety concerns such as carcinogenic food additives, as well as nutrition concerns such as diabetes and heart diseases. These are risks related to cumulative, whole-diet consumption. Finally, we examine broad food safety, which includes cradle-to-grave food safety risks such as agricultural water pollution, food waste, food packaging waste, and farmworker pesticide exposure. Typically, broad food safety concerns are considered questions of environmental protection and workplace safety.
Although some aspects of what we call intermediate and broad food safety receive regulatory attention under the monikers of nutrition, environmental protection, and workplace safety, narrow food safety dominates as a regulatory priority for the FDA and across federal law. Table 1 (below) maps the three types of food safety onto traditional areas of food policy.
1. Narrow Food Safety: Acute Ingestion-Related Risks
Narrow food safety focuses on acute ingestion-related risks that comprise the core of traditional food safety. Specifically, narrow food safety risks include microbial and chemical contamination that result in immediate health consequences, like foodborne illness or food poisoning.
The substantive provisions of the FDCA focus the FDA primarily on narrow food safety. In general, the FDCA directs the FDA to ensure against the “introduction or delivery for introduction into interstate commerce of any food, drug, device, tobacco product, or cosmetic that is adulterated.”[16] Thus, the bulk of the FDA’s food safety functions are linked to its authority over adulteration.
The FDCA identifies a variety of types of adulteration, and, although the statute does not expressly limit adulteration to narrow food safety, many of the specific types are so limited. The FDCA’s first definition of adulteration hinges on whether food includes a “poisonous or deleterious substance” that is “injurious to health.”[17] The statute governs both intentional additives, which we consider in Part I.A.2 below, and accidental additives, including both microbial contaminants and other non-food contaminants, such as mold, rodent filth, and insect parts.[18] For many of these accidental contaminants, particularly those that generate disgust but not actual health risks, the FDA sets informal tolerance levels, above which a food would be considered adulterated.[19] The FDCA also considers food adulterated “if it has been prepared, packed, or held under insanitary conditions . . . whereby it may have been rendered injurious to health.”[20] Prior to the passage of the FSMA, this sanitation provision was the primary way of addressing microbial contaminants.[21]
Similarly, the FSMA emphasizes the importance of protecting consumers from “exposure to an article of food . . . [that] will cause serious adverse health consequences or death to humans or animals.”[22] The FSMA expanded the FDA’s power over food processing and, for the first time, gave it express authority to regulate agriculture.[23] The statute directs the agency to prescribe best practices for farming and food processing.[24]
To implement the FSMA, the FDA focuses on sterilization of food growing and processing environments.[25] In 2015, the FDA finalized Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption (the “Produce Safety Rule”).[26] The Produce Safety Rule focuses on six potential sources of contamination: “soil amendments, [worker] hygiene, packaging, temperature controls, animals in the growing area, and water.”[27] For each source, the Rule identifies a variety of steps that farmers should take to prevent contaminants from coming into contact with raw produce. For instance, employers must provide employees with sanitary bathroom and handwashing facilities,[28] and farmers must take all reasonable measures to “exclud[e] domesticated animals from . . . enclosed facilities where covered produce, food contact surfaces, or food packing material is exposed.”[29]
The FDA promulgated a variety of other rules under the FSMA, including the Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food (the “Preventive Controls Rule”), which was finalized in 2015. The Preventive Controls Rule requires food processors to adopt a series of specified best practices and to develop food safety plans based on site-specific risk assessments.[30] Taken together, the FDA’s mandates under the FDCA and the FSMA direct agency focus and resources towards the narrow food safety goals of eliminating adulteration and accidental food contaminants.
2. Intermediate Food Safety: Cumulative Ingestion-Related Harm
Poor diet poses long-term health risks, including heart disease, hypertension, diabetes, and certain types of cancer;[31] such risks constitute intermediate food safety. This category of food safety is concerned not with the impacts of individual food choices in isolation (e.g., one bad bunch of lettuce that immediately makes you sick), but with an individual’s entire diet over time (e.g., overall sugar consumption and its impact on long-term health). The main intermediate food safety concerns are cancer and other health effects from cumulative consumption of food additives and diet-related disease from long-term consumption of unhealthy foods. Although regulation regarding additives is generally less strict than regulations governing accidental contaminants, the FDA treats it as a food safety issue.[32] In contrast, diet-related disease is typically not considered a food safety issue.
The FDA has a direct legislative mandate to regulate food additives.[33] The FDCA defines “food additive[s]” as “any substance the intended use of which results or may reasonably be expected to result, directly or indirectly, in its becoming a component or otherwise affecting the characteristics of any food.”[34] Food additives are prohibited unless the FDA expressly promulgates a regulation laying out the conditions under which each individual additive may be safely used.[35] The Delaney Clause in the Food Additives Amendment of 1958 prohibits the FDA from declaring an additive “safe” if it is carcinogenic.[36]
In practice, however, the FDA’s food additive regulation is relatively weak because substances that would otherwise be additives are exempt if they are “Generally Recognized As Safe (GRAS).” An additive is GRAS if it is
generally recognized, among experts qualified by scientific training and experience to evaluate its safety, as having been adequately shown through scientific procedures (or, in the case of a substance used in food prior to January 1, 1958, through either scientific procedures or experience based on common use in food) to be safe under the conditions of its intended use.[37]
The FDA’s GRAS notification procedure, finalized in 2016 but generally in place since 1997, allows companies to voluntarily notify the FDA of their own GRAS designations, without FDA oversight of the scientific procedures used to assess product safety.[38] This process has been subject to frequent criticism. One recent study estimated that food manufacturers have self-declared around 1,000 additives as GRAS without any disclosure to the FDA.[39] Another study concluded that of the 10,000 allowed food additives, about 3,000 have never been reviewed by the FDA either because they were self-affirmed or because they were determined GRAS by an industry trade association expert panel.[40] Several self-proclaimed GRAS additives were later banned from the food supply.[41] Litigation filed against the FDA in 2017 asserted that the FDA is abdicating its duties under the FDCA’s Food Additives Amendment by allowing the use of GRAS substances without premarket testing.[42]
The second significant category of intermediate food safety is diet-related disease. Today, much of the disease burden in the United States is linked with diseases caused by or correlated with diet, including type 2 diabetes, heart disease, stroke, hypertension, and various cancers.[43]
FDA regulation of healthy diets differs from its regulation of microbial contamination both in the scale of resources brought to bear and in the nature of the regulation.[44] The FDA regulates microbial contamination with express prohibitions on adulteration and prescriptive requirements related to food production, processing, and handling.[45] The FDA occasionally uses food additive regulations to address diet-related disease. Typically, however, such efforts are painstakingly slow, as demonstrated by recent regulatory battles over partially hydrogenated oils (PHOs) and sodium. PHOs contain large amounts of trans fats. Despite studies showing that banning trans fats could prevent 30,000 to 100,000 deaths[46] and 72,000 to 228,000 heart attacks,[47] the FDA took more than a decade to act with regard to PHOs.[48] In 2004 and 2009, the FDA received citizen petitions asking the agency to ban PHOs, which were then considered GRAS. In 2013, the agency was sued for its failure to respond to the 2009 petition.[49] In 2015, the FDA finally completed its review and revoked GRAS status for PHOs.[50] The 2015 final rule set a compliance date of June 18, 2018 for removal of trans fats, but the FDA extended that deadline for certain uses of PHOs.[51]
A similar process, kicked off by a 2005 citizen petition and 2015 litigation,[52] led the FDA to release voluntary guidance for the reduction of sodium in commercially processed foods, due to its contribution to hypertension and heart disease.[53] Although the FDA could mandate sodium reductions under its power to prohibit or set tolerances for additives,[54] it has not yet done so. These examples show that FDA has been hesitant to use food additives regulation to address nutrition concerns.
More commonly, the agency regulates healthy diets using public education, labeling, and other forms of information regulation. These provisions, including calorie labeling on menus and packaged foods, are knowledge-promoting rather than directly safety-promoting.[55] Although some FDA labeling regulations relate to acute health risks (such as allergens) and some to economic harms (such as fraud), over time, an increased portion relate to nutrition.
The FDA regulates nutrition information under the auspices of its authority over misbranding.[56] The 1990 Nutrition Labeling and Education Act (NLEA) expanded that authority to require the agency to promulgate regulations governing newly-mandatory Nutrition Facts Panels.[57] NLEA provisions differ from prior FDA authorities, which focused almost entirely on traditional food safety, because of the NLEA’s stated focus on “healthy dietary practices.”[58]
Although nutrition labeling has been strengthened in recent years, it continues to be the subject of much criticism. In recent updates to the Nutrition Facts Panel, the FDA announced that packaged foods manufacturers must separately list added sugars on nutrition labels, based in part on health recommendations from the American Heart Association, the American Academy of Pediatrics, the Institute of Medicine, and World Health Organization.[59] Even this new requirement, however, came only after years of community advocacy.[60] Further, the FDA faces widespread criticism for its failure to enforce labeling and misbranding regulations,[61] and for gaps in its labeling regulations.[62] Thus, although the FDA engages in nutrition regulation, its efforts focus on education and information disclosure and are not responsive to the full scope of nutrition-related intermediate food safety risks.
Nutrition regulatory programs at the USDA and elsewhere at the Department of Health and Human Services (HHS) track a similar pattern, focusing primarily on education. For instance, every five years, the Secretary of Health and Human Services and the Secretary of Agriculture must together publish a set of dietary guidelines that form the basis of federal nutrition education.[63] Based on recommendations from the Dietary Guidelines Advisory Committee, these guidelines are used to establish requirements for federal food service operations in publicly operated cafeterias.[64] The Food and Nutrition Service (FNS), within the USDA, is also responsible for using the Dietary Guidelines for Americans as the basis for the nutrition standards for the National School Lunch and School Breakfast Programs, which FNS designs and implements.[65]
In sum, both food additives and whole diet consumption patterns raise intermediate food safety risks. Federal regulators treat the former but not the latter as a food safety concern. Because of the GRAS exception, however, food additives regulation is weaker than narrow food safety regulation. The regulatory response to whole diet nutrition concerns is even weaker. Regulations for foodborne illness use a command and control approach, but lawmakers instead address whole diet nutrition concerns primarily through information regulation.
3. Broad Food Safety: Beyond Ingestion
Beyond the direct ingestion-related food safety concerns described in the previous two Sections lies a broad swath of other food-related public health risks. Food products have long lifespans and can generate public health costs both before and after consumption.
Prior to consumption, food production implicates health risks due to agricultural air and water pollution. Nitrates and other agricultural pollutants contaminate drinking water.[66] Toxic algal blooms caused by agricultural fertilizer runoff also threaten drinking water supplies.[67] Hydrogen sulfide and other air pollutants, including ammonia and particulate matter, threaten communities in the vicinities of large feedlots and other industrial-scale farms.[68]
Field workers face risks related to exposure to pesticides and other agricultural chemicals.[69] Although available data is imprecise, physicians diagnose between 10,000 and 20,000 pesticide poisonings each year among agricultural workers.[70] Workers in animal feeding and processing facilities often face risks related to unsanitary conditions and working with “sharp tools and heavy machinery, at high speeds.”[71] In addition, more than two million Americans are sickened annually by antibiotic resistant infections, resulting in at least 23,000 annual deaths.[72] Studies link the use of antibiotics in livestock raised for meat to antibiotic resistance in humans.[73]
After consumption, food continues to generate public health harms related to food and food packaging disposal.[74] Some estimates suggest that as much as 40 percent of food is wasted.[75] The environmental costs of food waste include resources wasted in producing food that is ultimately thrown away and methane emissions from the decomposing food itself.[76] Food packaging is also an issue of concern. A significant amount of food packaging is made from petroleum-based plastics, which break down after disposal into micrometer-sized particles that can make their way into the food chain as they are ingested by fish, invertebrates, and microorganisms.[77] About half of all plastics contain hazardous ingredients such as carcinogens and hormone disrupters, and other plastics can become toxic by absorbing these pollutants from the environment.[78] Plastic disposal, particularly in municipalities with solid-waste incinerators, can also impede air quality.[79] Finally, plastic production is resource intensive and environmentally hazardous.[80]
In addition, the food system also generates indirect public health costs related to greenhouse gas (GHG) emissions and resource use. Agriculture alone is responsible for almost 9 percent of US GHG emissions;[81] the food system as a whole is responsible for 19 to 29 percent of global anthropogenic GHG emissions.[82] The food system is also extremely water intensive; about 29 percent of the global human water footprint is attributable to production and consumption of meat and milk products,[83] and agriculture contributes to between 80 and 90 percent of the United States’ consumptive water use.[84] These footprints generate severe, though difficult to quantify, public health costs.[85]
These food production environmental externalities are under-regulated. The FDA rarely considers them in its regulatory processes, and it certainly does not regulate them directly. The EPA likewise under-regulates in this area. Although a patchwork of regulations exists, federal environmental law generally treats food and agriculture with a light touch, particularly on the topics of pesticide use and of water pollution from the largest animal farms.[86] The field of environmental regulation of the agricultural system is dominated by green payment programs, which pay farmers to adopt more environmentally sensitive agricultural practices, and eco-labeling schemes, which allow food sellers to make environmental claims on their labels if the products meet certain production criteria.[87] Participation in these programs is entirely voluntary, and ongoing levels of environmental harm, discussed earlier in this Section, demonstrate their inadequacy. The same under-regulation pattern repeats in the context of workplace protection for food and agriculture workers.[88]
The FDA regulates food packaging as a “food contact substance.”[89] Pursuant to the FDCA, the agency treats “food contact substance[s]” as food additives and requires that before a manufacturer introduce a new substance it either seek prior approval or notify the agency.[90] A notification must include a statement of intended use and a determination that the substance is safety for that use.[91] In evaluating new “food contact substances,” the FDA considers disposal concerns, but it has never denied an approval on this ground.[92] Disposal-related safety concerns highlight the narrow temporal nature of the FDA’s food safety mandate. By focusing on the immediate consequences of ingestion, the safety mandate misses the full range of potential long-term, persistent environmental consequences of food packaging materials.
In sum, food-related risks extend well beyond those associated with eating contaminated food. All three categories of food safety generate public health concerns, yet narrow food safety is the subject of much more sustained and systematic regulatory attention. By comparison, regulation in the other areas of food safety is frequently less prescriptive and is more information-based or voluntary. Where prescriptive regulation exists, it is often underenforced.[93]
The next Section offers a variety of hypotheses for the dominance of narrow food safety as a food system health concern. In Part II, we illustrate the health costs generated by the use of the narrow definition of food safety, explaining how this definition undermines the overall goal of reducing food-related health costs.
B. The Tendency Toward Narrow Food Safety
Given the broad range of risks associated with food production and consumption, why is our regulation of food safety so limited? We start with the premise, introduced in Part I.A and further developed in Part II, that this is not merely the result of operating regulatory siloes in which narrow food safety is addressed within one regulatory category and the other categories of food safety are addressed in other places. Instead, narrow food safety gets more robust and comprehensive regulatory attention than the other categories, in large part because of how Congress and the FDA have defined food safety. This Section offers a range of hypotheses grounded in the history of food safety law, the sociology of public fears about food safety, and the motives of the regulated industry. Regulation of traditional food safety in general (including food additives), and narrow food safety in particular, is more appealing to lawmakers and more palatable to the regulated industry than is regulation of intermediate or broad food safety.
1. Behavioral Economics and the Reactive History of Food Safety Law
Over the last hundred years, food safety laws have developed as a series of congressional reactions to specific incidents of poisoning and outbreaks of foodborne illness. This history of reactivity is unsurprising when viewed through the lens of behavioral economics.[94] Foodborne illness outbreaks are high-salience, low-probability events that generate disproportionate fear, and thus disproportionate regulatory response; by contrast, health risks associated with nutrition or the environmental footprint of the food system are often low-salience events that fail to generate robust regulatory response.[95]
The modern era of food safety law began in 1906, following publication of Upton Sinclair’s The Jungle.[96] The book’s powerful imagery of food contamination and unsanitary conditions in Chicago’s meatpacking district helped spark the passage of the Pure Food and Drug Act of 1906 and the Federal Meat Inspection Act of 1906.[97] Prior to The Jungle’s publication, Congress had been stalled for nearly thirty years on the passage of similar legislation.[98]
A public health disaster related to drug regulation prompted passage of the next major law regulating food and drug safety. In 1937, a drug called Elixir Sulfanilamide caused over one hundred deaths, and many children were among those killed.[99] Public outrage motivated the Food Drug and Cosmetic Act of 1938.[100] Fears of chemical use in food production also drove the Food Additives Amendment in 1958.[101] A statement submitted by the Cooperative League during hearings on that legislation illustrates these concerns. The witness warned of modern bakers “pull[ing] the wool over [a grandmother’s] eyes, when they convinced her that real white bread was better. They didn’t tell her that in order to get it white, they used a poisonous substance which gave dogs fits.”[102] Again, public response to acute poisoning and illness helped justify expansion of regulatory authority. The FDA’s own website describes many of its key legislative grants of authority regarding food, drugs, and medical devices as responses to outbreaks or injuries.[103]
The most recent congressional grant of regulatory authority continues this trend of reactivity. Congress enacted the FSMA partially in response to high-profile outbreaks of foodborne illness in foods such as spinach and eggs, and the primary goal of the statute is to improve the FDA’s ability to prevent future outbreaks.[104] The FSMA is the largest reform of federal food safety regulation since the 1930s.[105] It provides the FDA with express regulatory authority to address foodborne illness from pathogenic microorganisms, an issue the FDA had increasingly addressed over the years under an outdated legislative framework created prior to the discovery of such contaminants.[106] The FSMA also provides the FDA with expanded authority to regulate food growing, harvesting, and other handling practices on farms.[107]
This statutory history demonstrates a consistent trend of reactivity that reflects the underlying behavioral economics of food safety. Food safety regulation follows public fear and outcry in response to serious outbreaks or exposés. This reactive nature of regulation is unsurprising, as foodborne illnesses and poisoning trigger a visceral response for many people.[108] In contrast, the risks of developing diet-related diseases, such as heart disease or diabetes, do not elicit a comparable regulatory reaction, even though those risks are now more prevalent and costly than narrow food safety risks.[109] This may result in part from “availability bias”; we estimate risk based on our ability to imagine examples of the risk materializing and actually resulting in harm.[110] Foodborne illness outbreaks are dramatic and highly publicized, often leading to significant declines in demand for associated foods.[111] For instance, a 2006 E. coli spinach outbreak led to a 12 percent decline in demand for spinach.[112] Our innate revulsion to the symptoms associated with foodborne illness, along with the extensive media coverage usually afforded to outbreaks, compels us to remember and fear foodborne illness.[113] Intermediate and broad food safety risks are less “cognitively ‘available,’” meaning that most people cannot recall these risks as readily as they can for foodborne illness, so we fear them less.[114]
Intermediate and broad food safety risks are also less connected to the act of eating. Though foodborne illnesses can typically be traced to a single meal, it is difficult to trace diet-related diseases to a single source because these diseases emerge from a constellation of potential sources over a long period of time.[115] Compared with outcomes that seem more certain and immediate—like contracting listeriosis from a contaminated melon—outcomes that are probable but distant, like developing diabetes from excessive consumption of soda over many years, are mentally assigned far too little weight.[116]
Further, our disproportionate cultural and political focus on narrow food safety perhaps stems from the fact that humans evolved to fear potentially contaminated food. Our ancestors associated new foods with high risks of illness, and humans today carry these genetic relics.[117] However, we tend to regard familiar foods as safe.[118] Our familiarity with many of today’s processed foods that are high in sugar or sodium leads us not to fear them.[119] The reverse is also true. Subjects view unusually shaped but otherwise-wholesome vegetables as riskier than vegetables that conform with shapes typically found in the supermarket, leading consumers to avoid purchasing or consuming imperfect vegetables.[120]
Diet-related disease does not generate the same fear. Humans did not evolve to fear the detrimental effects of excess weight; energy accumulation instead may have arisen as an evolutionary adaptation among hunter-gatherers during times of feast and famine.[121] Overconsumption was not a widespread source of risk until relatively recently.[122] Some scientists suggest we may have a genetic predisposition to gorge on available food.[123] This evolutionary history may help to explain our difficulty in recognizing the long-term health hazards associated with foods that we encounter regularly and that bring us immediate pleasure. Here, another cognitive bias comes into play. Many people tend to overvalue short-term payoffs, like the joy of eating a hamburger, fries, and milkshake, and undervalue the long-term costs of repeatedly indulging.[124] This is particularly true in the realm of dieting: we plan today to diet tomorrow, but when tomorrow comes, we prefer to overeat for one more day.[125]
To be sure, diet-related disease currently has more salience than ever before. Dieting and healthy eating constitute a multi-billion-dollar industry, and concerns about nutrition are widespread and growing.[126] Nevertheless, perhaps because diet-related harms unfold in slow motion, they remain less of a catalyst for regulatory action than do narrow food safety concerns. They are also less likely to generate aggressive regulatory action, perhaps because consumers perceive diet as within their control and requiring less regulatory intervention; whereas they perceive narrow food safety as out of their control.[127]
The same complicated causal chains that make it harder for consumers to accurately assess the risks of diet-related disease and the environmental harms of food production also limit the capacity of lawmakers to develop easily implemented policy responses to those long-term risks. For policymakers, addressing foodborne illness may be more rewarding because the necessary measures may appear more straightforward and easier to implement, enforce, and measure than those required to effectively address issues of intermediate and broad food safety.[128] At the same time, foodborne illness may be easier to address politically because it does not generate the same concerns about paternalism that mire efforts at nutrition regulation.[129]
In sum, for both consumers and lawmakers, foodborne illness is a high priority that generates significant regulatory activity because it is perceived as presenting a greater risk and is an easier problem to solve.
2. Political Economy of Narrow Food Safety
We draw an additional set of explanations for the scope of narrow food safety regulation from analysis of the political economy of the food system. The interests of powerful food system players, including large food producers, processors, distributors, and retailers, drive the focus of policy makers. First, we identify a number of reasons why those industry interests align with narrow food safety regulation but discourage robust regulation of other food safety types; these include (1) the existence of private governance mechanisms, and (2) a scheme of tort liability for narrow food safety harms. Second, we examine the role of socioeconomics, concluding that the distribution of the benefits and burdens of narrow food safety regulation versus other types of food regulation reinforces the trend of heightened interest in narrow food safety.
To illustrate the political economy of food safety, we focus on the FSMA. Regulation of narrow food safety stands in stark contrast to regulation of other aspects of food production, processing, and distribution. Given the food and agricultural industry’s success in thwarting more robust regulation of everything from marketing targeted at children to agricultural water pollution, why was Congress able to pass the FSMA, which dramatically expanded the FDA’s authority over food and agriculture? Why, more specifically, did industry groups support the passage of the law? Two key structural issues explain industry support for the FSMA. The first is the role of private governance. The second is background tort law.
First, though the FSMA gave the FDA express authority over food safety procedures on farms for the first time, many farmers were already subject to private produce safety requirements in the form of buyer-imposed produce safety standards.[130] For instance, many farmers already participated in the USDA Good Agricultural Practices (GAP) auditing program.[131] In 2010, over one thousand farms in the United States were already GAP certified for the production of a fresh fruit or vegetable now regulated under the proposed produce safety rule, and the FDA acknowledged that many other farms could already be adhering to the GAP guide.[132] Although the USDA program was legally voluntary, many large-scale retailers required their suppliers to comply with the guide.[133]
In addition, private governance of industry players prior to the FMSA included product-specific marketing agreements that specify safety standards and inspections, again making industry support for the FSMA unsurprising. For example, over 90 percent of tomatoes grown in California are subject to a Tomato Audit Protocol, a set of food safety standards followed by the California Tomato Farmers.[134] The FDA also found that in 2010, approximately 80 percent of mushrooms grown in the United States complied with mushroom-specific good agricultural practices (M-GAPS)—a program developed by the American Mushroom Institute and Penn State University.[135] Other commodity-specific guidance exists for melons, green onions, citrus fruits, strawberries, apples, peppers, almonds, and avocados.[136] Finally, leafy green marketing agreements (LGMAs) in California and Arizona cover a significant percentage of the leafy greens grown in the United States. The Arizona LGMA alone covers about “85 percent of leafy green products consumed in the United States and Canada from November to March.”[137]
This widespread compliance with pre-existing private food safety governance generated support for the FSMA.[138] Many of the growers subject to these requirements prefer uniform national standards, which would require any farmers not already subject to private governance to play by the same rules, and could reduce individual compliance costs by ensuring that a single farmer would not be subject to different sets of requirements from different buyers.[139] Parallel private standards in the areas of nutrition, environmental protection, and workplace safety are less comprehensive or nonexistent.[140] Thus, the same pre-existing set of standards and protocols that led industry players to support narrow food safety regulation does not exist in the intermediate or broad food safety realms, explaining one rationale for the strong industry opposition to any increased regulation in those arenas.
The second structural issue explaining industry support for the FSMA is that narrow food safety, unlike intermediate or broad food safety, can readily generate tort liability. Food producers face strict tort liability for introducing microbial contaminants that make people sick.[141] By contrast, food producers who generate environmental harms, or who produce foods that contribute to diet-related diseases, rarely face any liability, let alone strict liability.[142] As a result, industry has more incentive to engage in risk prevention for narrow food safety than for any other form of food safety.[143] Another way to think about this is that narrow food safety harms are not externalities. Broad food safety harms always are. Intermediate harms are also externalities because the harms are not specific to individual transactions; instead they are the result of a large number of transactions over a long period of time.
Socioeconomics may also help explain the regulatory attention paid to narrow food safety. Foodborne illness is not uniformly correlated with wealth.[144] Indeed, foodborne illnesses like Campylobacter and Salmonella are often more prevalent among higher socioeconomic classes.[145] On the other hand, listeriosis is more common among those from lower socioeconomic classes.[146] The literature suggests that any potential relationship between socioeconomic status and incidence of foodborne illness is complex. By contrast, research has established more firmly that individuals from lower socioeconomic classes face higher prevalence of diabetes and other diet-related disease.[147] Similarly, the costs of food production’s environmental impacts, particularly those related to concentrated animal feed operations (“CAFOs”), are often borne by low-income communities and communities of color.[148] Although these statistics do not definitively explain why food safety has been defined narrowly, they are nevertheless a critical part of the story. No one is safe from foodborne illness. Larger constituencies, including those with more economic and political clout, advocate for laws regulating narrow food safety, while intermediate and broad food safety receive less robust and less influential public support.
For all of these reasons, although food generates risk before, during, and after ingestion, acute ingestion-related risks are easier and more politically palatable to regulate. It is worth noting that in recent years, nutrition has become a more salient issue, and many food companies have invested in nutrition-related product reformulation and marketing.[149] Although this is a significant market trend, it has not resulted in substantial regulatory change.[150] As the next Part illustrates, the strength of regulation and deployment of resources to narrow food safety still far exceeds investments made in intermediate or broad food safety. Furthermore, the next Part will highlight that beyond just disproportionately investing in narrow food safety, such investments in fact continue to undermine all areas of food safety.
II. The Costs of Narrow Food Safety
The traditional definition of food safety and the prioritization of narrow food safety over other aspects of food-related public health are problematic. First, the definition contributes to a misallocation of regulatory resources. Although microbial contamination results in a substantial number of deaths and illnesses every year, these numbers pale in comparison to deaths and illnesses related to intermediate and broad food safety. By defining food safety narrowly, regulators exclude these other costs from regulatory analyses. This exclusion then shapes how resource-limited agencies establish priorities. Part II.A compares the relative costs of traditional food safety and nutrition with actual allocation of regulatory resources. We look both at actual agency spending and at the types of regulatory tools used by the FDA and, where applicable, the USDA.
Second, a myopic focus on narrow food safety can have unintended consequences, because narrow food safety policies are often developed and implemented with minimal regard to intermediate and broad food safety. Put simply, regulating narrow food safety can worsen intermediate or broad food safety risks, leading to net negative health impacts. Part II.B illustrates several examples where this is the case.
Finally, a myopic focus on narrow food safety may actually make it more difficult to achieve narrow food safety. Standard approaches to narrow food safety emphasize prevention at the point of contamination. Part II.C observes that a systemic approach may more effectively protect narrow food safety by addressing underlying origins of risk and risk-factor multipliers that narrow food safety regulation currently misses. This Part concludes that defining food safety narrowly undermines both the efficiency and the effectiveness of our food safety regulatory apparatus.
A. Resource Allocation: Traditional Food Safety Versus Nutrition
It is perhaps one of the most obvious and repeated tropes of administrative law that, in a limited-resource world, public expenditures to promote health and welfare must be prioritized. We begin with the basic assumption that a primary goal of any prioritization process should be to maximize the number of lives saved. Although this Section does not engage in a precise cost-benefit analysis, it takes a preliminary look at the costs and benefits of investment in Food System Safety and asserts that the current balance of expenditure tilts too strongly in favor of traditional food safety; as a result, it draws needed resources, including policymaker attention and regulatory enforcement capacity, away from food system risks that are objectively costlier and more harmful. We focus on a direct comparison between nutrition and traditional food safety because data on regulatory expenditures and public health costs in these areas is more readily available, but we hypothesize that a comparison across all categories of Food System Safety would support our conclusion that the scale of investment in traditional food safety relative to investment in other categories is not rational based on the associated harms.
We begin with a preliminary assessment of the relative severity of foodborne illness risks as compared to nutrition risks. Foodborne illness is a significant public health concern with substantial costs. Such illnesses annually sicken about 48 million Americans, resulting in 128,000 hospitalizations and 3,000 deaths.[151] Estimates of associated costs range from $14.1 to $152 billion annually.[152]
Diet-related disease is even more deadly and costly. In 2016, heart disease alone caused over 635,000 deaths in the United States, more than 200 times higher than that of foodborne illness.[153] In 2014, diabetes killed more than 80,000 Americans.[154] A growing number of Americans, nearly 10 percent, already suffer from type 2 diabetes, and one-third are pre-diabetic.[155] Obesity increases the risk of heart disease and diabetes,[156] and in 2014, the National Institutes of Health estimated that 70.2 percent of the population was either overweight or obese.[157]
The economic costs of these diet-related diseases are staggering. In 2017, diabetes alone imposed $237 billion in medical care costs and an additional $90 billion in lost productivity.[158] Heart disease cost $199.2 billion in medical costs, and $130.5 billion in lost productivity, and these numbers are only projected to rise.[159] The American Heart Association predicts that by 2030, total direct medical costs for heart disease will be $918 billion, and lost productivity costs will be $290 billion (in 2012 dollars).[160]
Table 2: Cost and Illness Comparison[161][162][163][164][165][166][167][168][169]
To assess resource allocation, we look first at actual regulatory expenditures.[170] To what extent is the federal government investing in each of these issues? Although the numbers available do not offer precise answers, they indicate overinvestment in narrow food safety relative to nutrition.
Analysis of the FDA and USDA budgets and related materials show significant financial investment in traditional food safety and much more limited investment in nutrition and diet-related disease. In 2016, the FDA spent nearly $1 billion on activities related to regulating the food supply.[171] A 2018 Government Accountability Office (GAO) report found that 98 percent of this budget was spent on traditional food safety and a mere 2 percent on nutrition.[172] This funding supported 4,200 full-time employees working on food safety, and only 97 full-time employees working on nutrition-related activities.[173] GAO found that between January 2011 and September 2017, the FDA released thirty-three “key proposed or final regulations”; of these, twenty-one were food safety-related; five were both nutrition- and food safety-related, and only seven were nutrition-related.[174] During that same period, the FDA also released 111 “key draft or final guidance documents”; of these, eighty-two were food safety-related; seventeen were related to both nutrition and food safety, and only twelve were nutrition related.[175] GAO also found that the FDA was unable to “fully assess progress toward its food safety- and nutrition-related goals” because it had “developed performance measures related to some, but not all, of the eight strategic objectives that support its goals.”[176] Notably, while the FDA had set performance measures for all but one of its five food safety-related objectives, it had not set performance measures for two of its three nutrition-related objectives.[177]
The FDA’s own descriptions of its priorities and activities reflect this assessment that nutrition is a lower priority for the agency. In its 2018 budget request justification narrative, the FDA characterized its priorities as “responding to outbreaks, working with industry to implement FSMA regulations, reviewing infant formula notifications, helping to ensure the safety of dietary supplements, conducting reviews of food ingredients and packaging, and ensuring that foods are safe and properly labeled.”[178] The FDA program description does specify: “The Foods Program ensures that . . . nutrition labeling is informative and accurate. The Foods Program also promotes a nutritionally healthy food supply.”[179] But only a handful of the specific 2016 accomplishments described in the narrative relate to nutrition.[180]
An analysis of the USDA’s budget repeats this pattern of prioritization of traditional food safety over nutrition. In 2016, the USDA’s Food Safety and Inspection Service (FSIS), which oversees traditional food safety for meat, poultry, and some egg and fish products, had a budget of $1.273 billion.[181] Quantifying USDA spending on nutrition is more challenging. This is because the USDA’s Food and Nutrition Service (FNS), the budget of which exceeded $100 billion in 2016,[182] primarily funds hunger relief rather than nutrition. The FNS is responsible for the USDA’s fifteen nutrition and food security programs.[183] The FNS’s leading budget item is the Supplemental Nutrition Assistance Program (SNAP) which provides eligible individuals with a financial benefit with which to purchase groceries.[184] SNAP cost more than $80 billion in 2016.[185] This spending is, however, nutrition indifferent; benefits can be spent on any food item, including soda, regardless of the nutritional benefit.[186] Even among programs that are not nutrition indifferent, the focus is on combating malnourishment rather than diet-related disease.[187]
A few specific USDA nutrition programs address whole-diet health, but spending for these programs hovers at around one-third the level of USDA traditional food safety spending. Most significantly, SNAP Education (SNAP-Ed) cost $414 million in 2017.[188] SNAP-Ed is a grant program that provides funding to states to create nutritional education and obesity prevention programs for SNAP participants and SNAP-eligible individuals.[189] Other programs include the Food Insecurity Nutrition Incentive, which awarded $16.8 million in 2016 to incentivize “purchases of fruits and vegetables” among SNAP recipients,[190] and the Healthy Food Financing Initiative, which awarded about $22 million in 2015 to invest in development of grocery stores, farmers markets, and other healthy food retail institutions in neighborhoods lacking food retail.[191]
These numbers suggest that although federal government investment in hunger relief is substantial, the scale of investment in nutrition improvement by the two main agencies, the FDA and USDA, pales in comparison to investment in traditional food safety. This discrepancy is particularly problematic when compared to the significant disparity in the number of individuals impacted. In other words, for traditional food safety, many more dollars are invested per life lost than for nutrition.[192]
In addition to the total number of dollars spent, we must also examine what dollars are spent on. Regulatory instrument choice serves as one indicator of the perceived severity of the problem. Here, the contrast between nutrition and traditional food safety is even starker. Typically, more serious threats justify more invasive regulatory methods. For instance, hefty “sin taxes” on cigarettes, which followed less successful education campaigns, are now largely uncontroversial because the dominant public perception is that cigarettes are extremely unhealthy and have no redeeming qualities.[193] By contrast, taxes on sugary drinks are highly controversial because the public has not widely accepted that soda is extremely unhealthy.[194] Many view a sugar-sweetened beverage tax as too paternalistic, and advocates focusing on consumer freedom have been extremely successful in swaying lawmakers and voters alike.[195]
Traditional food safety regulation includes a broad array of prescriptive, command-and-control regulatory programs. Both the USDA and the FDA set specific standards defining adulteration, inspect facilities for compliance, mandate recordkeeping, and exercise their authority to prohibit adulterated products from entering the stream of commerce.[196]
By contrast, the FDA’s approach, and the approach across the federal government to addressing diet-related disease, uses a much lighter touch. Components include limited requirements related to labeling for nutrition-related purposes, funding for research and education, and most recently, standards for voluntary reduction of ingredients with long-term potential harm, such as the FDA’s voluntary guidance on sodium reduction.[197]
The contrast between prescriptive regulations on the one hand, and education or voluntary standards on the other, reflects a serious mismatch between the nature and severity of each problem and the solutions brought to bear. Taken together with the discrepancy in the resources allocated to each of these types of food safety, the disparity in the strength of the regulatory methods used illuminates the depth this mismatch. The next two Sections show how, beyond mere misallocation of resources, the focus on narrow food safety has unintended consequences, sometimes increasing overall food system health effects by worsening outcomes in intermediate and broad food safety (Part II.B), and sometimes limiting the effectiveness of the regulations intended to reduce narrow food safety risks (Part II.C).
B. The Collateral Consequences of Prioritizing Narrow Safety
The regulatory focus on narrow food safety can worsen other types of food safety risks. Prioritizing narrow food safety over other food-related safety concerns can generate a variety of unintended consequences, including both short-term and long-term health and environmental tradeoffs. Although it is difficult to quantify these tradeoffs, examples from the FSMA and the FDCA demonstrate how tradeoffs may occur. We show that these tradeoffs exist and that the FDA frequently affords them insufficient attention.
“Health-health tradeoffs” are a common phenomenon in risk regulation.[198] These occur when regulatory actions designed to resolve one kind of safety problem generate another kind of safety problem.[199] In the food safety context, a quintessential example is the use of nitrates to process foods. Nitrates reduce the risk of botulism in cured meat products.[200] But there is some concern that once added to foods, nitrates react with other ingredients to form carcinogenic compounds.[201]
In this example, both risks fall within narrow food safety; where narrow food safety conflicts with other intermediate or broad food safety risks, engaging in risk tradeoff analysis is even more challenging. Division of labor among federal agencies contributes this problem.[202] Actions required or encouraged to reduce instances of foodborne illness, such as excluding wildlife from produce fields or shifting to single-use packaging, have ancillary consequences that extend beyond the traditional domain of the FDA.[203] The remainder of this Subsection identifies several examples of such tradeoffs. Underlying each of these examples is an empirical question about the precise value of the tradeoff that we do not purport to answer.[204] Instead, we intend this discussion to raise the possibility that a single-minded emphasis on narrow food safety may have costs to intermediate and broad safety that are not outweighed by the accompanying gains in narrow food safety.
1. Conflict Between Narrow and Intermediate Food Safety
The FDA’s focus on narrow food safety presents tradeoffs with nutrition. The FSMA grants the FDA the express authority to regulate on-farm practices to reduce the risks of foodborne illness in produce.[205] Under this statutory mandate, the FDA promulgated the “Produce Safety Rule,” which imposes a large regulatory burden on producers.[206] While improving narrow food safety, implementation of this rule also has the potential to increase intermediate food safety risks by decreasing the availability of produce and increasing the cost of growing fruits and vegetables.
Diet-related diseases have many causes including genetic predisposition and physical activity levels, but diet—specifically the overconsumption of unhealthy foods and the underconsumption of healthy foods like fruits and vegetables—is a particularly important factor.[207] World sugar consumption has tripled in the past fifty years.[208] At the same time, calorie availability per capita of fruits and vegetables has held relatively steady.[209] One well-documented challenge in consuming a healthy diet is the comparative cost of purchasing processed food items as compared to fruits and vegetables.[210] In the United States, between 1985 and 2000, the inflation-adjusted price of fresh fruits and vegetables rose by 39 percent; and the price of carbonated soft drinks fell by nearly 24 percent.[211] In addition to purchase prices, fruits and vegetables carry additional costs such as electricity and gas costs for food storage and preparation; expenses for purchasing cooking appliances; time and knowledge required for preparation; and higher waste as produce spoils more quickly than processed products. For many Americans, demand for food products is cost-dependent.[212]
At the same time, the United States undersupplies produce: according to a 2006 study, the United States produced 24 percent fewer servings of vegetables than it would need if every American were to eat the recommended servings under the Dietary Guidelines for Americans.[213] The FSMA may exacerbate this problem. The FDA’s Final Regulatory Impact Analysis for the Produce Safety Rule acknowledges that for some farms, the costs of compliance might “halt[] production of the crops . . . deem[ed] too costly to grow, pack, harvest, and hold.”[214] In response to commenters who feared that the Produce Safety Rule would “reduce access to . . . healthy food,” the FDA engaged briefly with the possibility that overall levels of produce production might decrease, but ultimately rejected this possibility, stating that it “does not believe that this rule will reduce access to produce.”[215]
Commenters also raised the related concern that producers might pass on increased production costs to consumers.[216] Increased produce costs might then reduce produce consumption levels. The FDA dismissed this concern by estimating that, because the total cost of the rule constitutes only about two percent of the value of produce sold in the US, any resulting price increase would be small.[217] This response fails to take into account the fact that increased production costs will not be evenly distributed: even though average price increases might be small, price increases for particular products or in particular regions might be much higher. In addition, this response ignores the possibility that for low-income consumers even very small price increases might be meaningful.[218]
The Produce Safety Rule thus may exacerbate diet-related disease risks both by increasing the costs of fruits and vegetables vis-à-vis processed products, and by decreasing the supply of produce. In estimating total costs of the rule, the FDA focused primarily on compliance costs and did not calculate any public health costs.[219] Yet, the costs excluded from the analysis have the potential to impact the most prevalent and costly diet-related risks, and if they had been included, may have painted a different picture of the regulation’s overall health impacts. With the fruit and vegetable supply already short, and diet-related diseases at all-time highs, even small changes in cost and supply of produce may have a big impact on public health.
2. Conflicts Between Narrow and Broad Food Safety
Standard approaches to narrow food safety have a variety of significant consequences for broad food safety. We focus here on two examples, increased food waste and increased plastic waste, but there are a variety of others including lost biodiversity, increased soil erosion and water contamination from agricultural runoff, and lost carbon storage.[220]
Generating Food Waste
One immediate consequence of narrow food safety regulation is food waste, which raises broad food safety concerns due to its environmental impacts. To be sure, removal of unsafe food from the human food stream is essential to human health, but the current approach to narrow food safety regulation has several unintended consequences. First, overzealous food safety regulation may lead to superfluous food waste. Second, food removed from the human food chain is likely to be thrown away, even when it can be safely repurposed.
One estimate suggests that every year in the US between 125 and 160 billion pounds of food are wasted;[221] this constitutes about 40 percent of the US food supply.[222] Wasted food impacts the environment in several ways. First, it contributes to a massive squandering of natural resources: in the US, roughly 20 percent of the freshwater, cropland, and fertilizers put toward agriculture are used to produce food that is wasted.[223] Food waste is the largest component of municipal solid waste that ends up in landfills and incinerators.[224] Food that decomposes in landfills produces methane, a “potent greenhouse gas” with 25 times the warming potential of carbon dioxide.[225] In total, wasted food produces at least 113 million tons of carbon dioxide equivalent.[226] There has been some federal response; most notably by the USDA and EPA.[227] In 2015, those two agencies jointly announced a National Food Waste Reduction Goal.[228] Until recently, the FDA has been conspicuously absent from efforts to prevent waste or ensure that discarded food is used. Yet the FDA could play a vital role, as much of this food is still safely edible and often goes to waste because of unclear rules for food donation.[229] In October 2018, FDA took an initial foray into this area, joining the EPA and USDA in signing a Memorandum of Understanding entitled “Winning at Reducing Food Waste.”[230] This led to the announcement in April 2019 that these three agencies will work together to implement a “Winning on Reducing Food Waste Federal Interagency Strategy.”[231] It still remains to be seen, however, what steps the FDA will take to implement this agreement.
The following examples from a range of food safety contexts illustrate how narrow food safety regulation generates food waste. First, FSMA regulations contain a variety of measures designed to ensure that animals do not introduce contamination into produce fields. Farmers must take all reasonable measures to “identify[] and not harvest[] covered produce that is reasonably likely to be contaminated with [animal excreta] . . . [or] that is visibly contaminated with animal excreta.”[232] Although the FDA drafted an environmental impact statement (EIS), and made some modifications to the rule to reduce its environmental footprint, the EIS does not adequately consider the potential food waste impacts of the rule.[233] The FDA also rejected waste-reduction alternatives, including proper washing of contaminated produce.[234] Although it is too early to determine exactly how much food will be wasted as a result of the new rule, anecdotal reports of the Leafy Green Marketing Agreement, a pre-FSMA produce safety agreement, suggest that field inspectors often require farmers to throw away all produce grown within a twenty-foot radius of the animal incursion.[235]
Second, the FSMA authorizes the FDA to instigate mandatory recalls.[236] The food waste consequences of recalls are serious.[237] Recalls lead to waste of the food item being recalled, and can often cause waste of items that are eventually found not to be the foodborne illness vector. For example, in 2008, the FDA warned consumers about a possible Salmonella outbreak in tomatoes.[238] Although the warning was later revoked, tomato demand declined, leading to more than 30 percent of US tomato acreage going unharvested that year.[239] Further, when a food product from one state or region is implicated in an outbreak, consumers often avoid the product entirely, even if it can be purchased from another region.[240] Although foodborne illness warnings and recalls are sometimes necessary, the FSMA gives no guidance to the FDA on balancing acute food safety with environmental concerns likely to result from unnecessary waste,[241] nor does the FDA embark on any comprehensive planning that could help divert or recover food that is a recall casualty. Both the FDA and the USDA, which governs meat recalls, provide guidance materials with very detailed requirements on the disposal and destruction of recalled products.[242] But with the exception of a brief note in the FDA’s investigations manual indicating that the agency must witness the “reconditioning or destruction” (emphasis added) of the product,[243] and one in the USDA Directive stating that the agency must receive prior notification of a recalled product’s “disposition . . . (e.g., destruction or relabeling)” (emphasis added)[244]—neither agency provides specific guidance, encouragement, or direction for how companies or individual consumers might relabel, recondition, donate, or otherwise use recalled products.[245]
Third, FDA inaction on food date labels also exacerbates food waste. Although “best before,” “use by,” “sell by” or other such labels typically serve only as quality or freshness indicators, many people assume that if such a date has passed, the food is unsafe and needs to be thrown away.[246] Food businesses, food recovery organizations, and food bank recipients are similarly confused, as are state regulators.[247] Because there is no federal law regulating date labels, states are free to pass their own date label regulations or requirements.[248] In some states, the sale or donation of past-date food is restricted or prohibited.[249] In fact, unnecessary waste due to date labels is so prevalent that one study concluded that simply standardizing food label dates and instructions was the most cost-effective approach to reducing US food waste; this reform could prevent 398,000 tons of food waste and provide $1.8 billion in economic value annually.[250] The FDA has a mandate to protect consumers from misleading labels,[251] but, despite evidence that these labels mislead businesses and consumers, the FDA has taken no regulatory action governing date labels.[252] Instead, in May 2019 the FDA Deputy Commissioner for Food Policy and Response published an open letter to industry encouraging use of the term “Best if Used By” for producers using a label to communicate product quality.[253] Ironically, the FDA has indicated reluctance to regulate in this area partly on the ground that dates are not safety-related.[254] The USDA also does not regulate date labels for meat and poultry products, the foods under its purview, but its industry guidance also recommends that manufacturers use the term “Best if Used By” if they are using a date label to indicate a product’s quality.[255] Because use of the term “Best if Used By” is recommended but not required, industry has a choice of whether to use this standard label, and in more than half of states, use of this standard language is not allowed due to state law.[256] Eating food past the date is not linked with narrow food safety risks,[257] but the waste that occurs due to confusion over the many labels contributes to environmental degradation and thus impacts broad food safety. The lack of required standard labels, motivated in part by FDA’s view of its regulatory mandate, contributes to ongoing confusion and waste, thus exacerbating these broader food system safety risks.
Generating Plastic Waste
A variety of food safety rules create preferences for single use packaging. Although none of the FDA’s rules expressly prohibit reusable packaging, they make the option more burdensome by imposing extensive requirements related to equipment selection and to sanitizing and washing procedures. Single use packaging has extensive environmental costs that are not fully accounted for in these regulations.[258]
The FDA Food Code illustrates the point. The Code is a model food safety regulation governing restaurants and other food service establishments that has been adopted at least in part by all fifty states.[259] The Code allows food to contact only certain types of surfaces: linens or other equipment laundered and sanitized pursuant to a lengthy list of requirements or “single service and single use articles.”[260] Similarly, while single use wipes can be used and then thrown away, cloths for wiping counters must be “held between uses in a chemical sanitizer solution.”[261] Other provisions simply permit the use of single use items even when other options are readily available. For instance, food employees “may not contact exposed, READY-TO-EAT FOOD with their bare hands and shall use suitable UTENSILS such as deli tissue, spatulas, tongs, single-use gloves, or dispensing EQUIPMENT.”[262] The Code goes on to allow employees to touch food with bare hands only after satisfying a long list of requirements.[263] Finally, with regard to customer refills using durable mugs, the Code prohibits food service establishments from doing so “except for refilling a CONSUMER’S drinking cup or container without contact between the pouring UTENSIL and the lip-contact area of the drinking cup or container[;] FOOD EMPLOYEES may not use TABLEWARE, including SINGLE-SERVICE ARTICLES, soiled by the CONSUMER, to provide second portions or refills.”[264]
FSMA rulemaking repeats this trend. The produce safety rule includes the general requirement that “[i]f you reuse food-packing material, you must take adequate steps to ensure that food contact surfaces are clean, such as by cleaning food-packing containers or using a clean liner.”[265] This provision, and others related to food contact surfaces and equipment sanitation, is excluded from the EIS, primarily on the ground that they are consistent with existing sanitation rules.[266]
Although it is difficult to quantify the precise effects of these preferences, a few statistics on overall use of disposables suggest the scope of the problem. On the consumer side, Americans use 500 million straws[267] and 100 million plastic utensils per day[268] and throw away 25 billion Styrofoam coffee cups per year.[269] On the distribution side, less data is available, but disposables are used throughout the supply chain for food production, processing, transportation, and preparation. Disposables include packaging to prevent damage in transit, cleaning materials, and disposable gloves used in food service.[270]
These disposables impose significant environmental costs including resource consumption and pollution. Resource consumption concerns relate to continued extraction of raw materials used to produce paper, plastic, and glass.[271] Pollution concerns relate both to proper disposal—air and water emissions from landfills and combustion facilities—and improper disposal—plastics in oceans and other waterways.[272]
The EPA recommends source reduction as a primary means for reducing the environmental footprint of food packaging.[273] But, as the examples above demonstrate, FDA regulations fail to incorporate this recommendation. Instead, those regulations incentivize disposables, prioritizing relatively low-probability but high-salience events (cross-contamination from durables) over high-probability but low-salience events (environmental effects of disposables).
C. The Self-Defeating Consequences of Prioritizing Narrow Food Safety
In addition to making tradeoffs in favor of narrow food safety that potentially worsen intermediate and broad food safety, the singular focus on narrow food safety can be self-defeating. The following examples demonstrate that federal agencies often miss opportunities to reduce acute food safety problems by failing to think more holistically about the food system. In these examples, the regulatory approach is narrow in different kinds of ways. Typically, it is narrow because it focuses almost exclusively on microbial contamination—zeroing in on adulteration without looking at how adulteration interacts with other food system issues—but it is often also narrow in its approach to risk assessment—focusing on the moment of microbial contamination rather than on the origins of the microbes. A common theme throughout this Section is the siloing of regulatory responsibility. The FDA has jurisdiction over a small range of food safety risks and over a small range of regulated entities. It is often not authorized to engage in such comprehensive risk assessment and regulation.
1. Diet-Related Disease and Susceptibility to Foodborne Illness
Under-regulation of nutrition risks heightens susceptibility to foodborne illness. Thus, high and growing rates of diet-related diseases themselves contribute to increased acute food safety risks.
Diabetes weakens the immune system, and food poisoning is especially likely to affect those with weakened immune systems. For this reason, the Mayo Clinic lists diabetes among the most serious risk factors for food poisoning.[274] The FDA warns those suffering from diabetes about their increased risk, noting
[a] consequence of having diabetes is that it may leave you more susceptible to developing infections—like those that can be brought on by disease-causing bacteria and other pathogens that cause foodborne illness. Should you contract a foodborne illness, you are more likely to have a lengthier illness, undergo hospitalization, or even die.[275]
Notably, according to the FDA, diabetes can damage the digestive tract, delaying digestion and allowing pathogens to remain in the system longer and multiply.[276] One study reported that diabetes patients were three times more likely than the general population to contract salmonellosis, four times more likely than the general population to contract campylobacteriosis, and twenty-fives times more likely than healthy people without diabetes to develop listeriosis.[277]
Cancer and cancer treatments also weaken the immune system. The Mayo Clinic includes those undergoing treatment for cancer among the groups most susceptible to foodborne illness.[278] The FDA itself also provides guidance on how those suffering from cancer or undergoing cancer-related treatments can reduce their risk of foodborne illness.[279]
As discussed in Part II.A, large numbers of Americans suffer from diet-related disease or are at risk for diet-related disease.[280] These Americans are at higher risk for contracting foodborne illness and suffer more severe consequences of foodborne illness when infected. This example demonstrates that under-regulation of intermediate food safety risks worsens narrow food safety risks.
2. Farm Environments and the Emergence of Foodborne Illness
Food handling practices on farms and at processing plants have significant food safety consequences. For instance, few measures are more important to preventing microbial contamination than those requiring employers to provide employees with sanitary bathrooms and handwashing stations.[281] Other measures, such as those requiring employees with illnesses that might be transmittable via food contact to stay away from food contact until recovered are common sense.[282] The FSMA is very successful in addressing these kinds of localized risk, but far less successful at addressing the broader risks of an industrialized food system.[283] By focusing narrowly on microbial contamination and on produce farms as the locus of risk prevention, Congress and the FDA miss systemic causes of risks, potentially making safe produce more costly and more elusive. As the following examples demonstrate, many of these underlying causes can be addressed through more comprehensive regulation of broad food safety. In the absence of such regulation, current approaches to food safety are in some cases ineffective or counterproductive.
From Feedlot to Farm
The FSMA’s focused approach on regulating produce farms fails to consider that produce farms are not always in the best position to mitigate contamination. Concentrated animal feeding operations (CAFOs), which consolidate meat production, generate large amounts of manure and thus microbial contamination.[284] To the extent that many CAFOs are unable to contain their waste, these microbes threaten both the meat supply and the produce supply.[285] Contamination may travel to a neighboring produce farm via either wild (or feral) animals or water runoff. In 2018, an outbreak of E. coli in romaine lettuce from Yuma, Arizona led to nearly 100 hospitalizations and five deaths.[286] The FDA found that canal water used for irrigation contained the same E. coli strain, and that the canal ran next to a CAFO that housed up to 100,000 head of cattle.[287] The FDA did not ultimately say the CAFO was the cause of the contamination because “samples collected at the CAFO also did not yield the outbreak strain,” yet it noted that while “[o]ther possible explanations for how the irrigation canal became contaminated are possible, . . . [it] found no evidence in support of alternative explanations.”[288] In cases such as these, produce farmers may not actually be the least cost avoiders of microbial contamination, but they bear the brunt of the regulations as the FDA has no authority to regulate CAFOs.[289] The FDA acknowledges that farmers downstream from CAFOs may need additional layers of testing and to install supplemental filtration for irrigation water.[290] A lack of regulation of broader food safety here allows activities upstream to worsen narrow food safety risks downstream.
Sterilizing Farm Environments
Narrow food safety regulation often relies on sterilization as a strategy to eliminate microbial contaminants. Sterilization strategies direct farmers and food producers away from more environmentally friendly practices that could promote broader food safety, and miss the ways that those practices could, in some cases, promote narrow food safety.
The FSMA emphasizes sterilization throughout both the Produce Safety and Preventive Controls rules. For instance, the Produce Safety Rule requires that any steps to treat agricultural water (water used for irrigation or produce washing) “be effective to make the water safe and of adequate sanitary quality.”[291] Both rules require that food contact surfaces be “sanitize[d],” which “means to adequately treat cleaned surfaces by a process that is effective in destroying vegetative cells of [pathogens], and in substantially reducing numbers of other undesirable microorganisms, but without adversely affecting the product or its safety for the consumer.”[292]
Although “sterilization” of food and food environments can mitigate microbial contamination, it misses an opportunity to “co-manage” food production and processing for general environmental and narrow food safety benefits.[293] The FDA defines “co-management” as farming “strategies [that] balance food safety concerns with environmental and farm management concerns.”[294] Co-management relies on the principal that microbially diverse environments offer better protection against harmful microbes. Some agrichemicals can increase prevalence of E. coli by decreasing predatory and competitor bacterial abundance.[295] Sterilization can also undermine food safety by reducing the presence of insects, yet these insects can be beneficial for food safety. For example, one recent study identified faeces-feeding beetles as a key ally for improving food safety.[296] Co-management would assess risk across the entire food system. It might, for instance, call for “coordinating management practices among feedlot operators, ranchers, and producer growers,” reducing runoff with secondary treatment wetlands, or planting produce that is not eaten raw in areas adjacent to grazeable lands.[297]
Advocates for co-management also call for sequestering pathogens by maintaining and installing vegetated buffers.[298] Historically, farmers have removed these buffers at the behest of food safety inspectors, operating on the theory that these buffers increase risk by attracting wildlife.[299] Although the FSMA does not include any specific requirements with regard to buffers, it creates incentives for farmers to remove buffers by prohibiting harvest of food if there is evidence of contamination from wildlife.[300]
In the Final EIS for the Produce Safety Rule, the FDA acknowledged that “the concept of co-management is important in promoting stewardship on the farm, including protecting water and soil quality and conserving wildlife and ecosystem habitat, while balancing food safety and farm productivity goals.”[301] The preamble to the final Produce Safety Rule also acknowledged the importance of co-management for environmental and food safety benefits, but the FDA declined to define co-management in the rule itself or to require any affirmative conservation-friendly practices.[302]
Antibiotic Resistance
Concerns about antibiotic resistance are on the rise, and a growing literature links such resistance to the high levels of antibiotic use in food-producing animals.[303] Estimates suggest that farms use 80 percent of all antibiotics sold in the United States.[304] Farmers give antibiotics to animals not just to treat illness, but also to prevent disease and promote growth.[305] These latter two uses often involve consistently giving animals low-dose “[s]ubtherapeutic” amounts of antibiotics over time.[306] The FDA’s under-regulation of antibiotics heightens narrow food safety risks by making foodborne illnesses resistant to antibiotics and thus more difficult to treat.
The FDA has authority over new animal drugs and animal feed containing new animal drugs.[307] The FDA may permit only those animal drugs that are safe for human health, and must withdraw approval for animal drugs if evidence emerges that they pose risks to human health.[308] The withdrawal process can be contentious and lengthy. The FDA has identified the cost and time of the withdrawal process as a key reason for its failure to withdraw approvals.[309] Many other countries have taken aggressive steps to reduce antibiotic use in farm animal production by banning or restricting antibiotic use for growth promotion,[310] yet the FDA has not withdrawn approval for the bulk of the antibiotics in use for farm animals.[311] Instead, it issued nonbinding recommendations encouraging manufacturers to stop marketing and farmers to stop administering antibiotics for growth promotion.[312] These documents encourage farmers to use antibiotics only for therapeutic uses (i.e., “uses that are considered necessary . . . for animal health”) under the oversight of a veterinarian.[313] In addition, the FDA updated its reporting system to get better annual data on sales and distribution of antibiotics for use in different species of animals.[314]
The ongoing use of antibiotics in animal production has huge implications for health and safety. Notably, the failure to regulate broader food safety here makes narrow food safety issues much worse. The Centers for Disease Control and Prevention (CDC) estimates that antibiotic-resistant bacteria and fungi sicken over two million Americans every year, resulting in more than 23,000 deaths per year.[315] According to the CDC, these antibiotic-resistant infections are particularly concerning because they require longer, more costly treatments than other foodborne illnesses and result in greater disability and death than infections that can be treated with antibiotics.[316] Further, if antibiotics do not work as well or at all, many common or routine illnesses or infections that we can easily treat today will, in the future, potentially be deadly.[317]
3. Food System Workers and the Spread of Foodborne Illness
More robust regulation of food system working conditions could also promote narrow food safety. In the absence of such regulation, narrow food safety rules can be self-defeating. Food workers are a source of contamination. As mentioned above, rules related to handwashing and excluding sick workers are common sense. Indeed, employee hygiene practices are one of the leading causes of foodborne illness.[318] But, implemented in isolation from consideration of worker protection, they can be counterproductive.
The FDA’s Preventive Controls Rule emphasizes the need to isolate sick workers from food production.[319] Similarly, the FDA Food Code requires that employees report specific illnesses, including hepatitis A and norovirus, even if there is a confirmed disease case in their household and the employee herself is not sick.[320] It also requires excluding symptomatic employees from food establishments, particularly those serving highly susceptible populations.[321]
Yet such rules, without parallel rules ensuring that hourly wage employees can get paid sick leave, gives workers strong incentives to hide their illnesses.[322] An employee who properly reports may have to miss twenty-four hours or more of work.[323] This example is different from the rest because it deals not with health risks but with economic justice.[324] But it follows the same pattern that narrow food safety is undermined by a policy that focuses on the direct cause of narrow food safety risk—employee food contact—and not on the structural feature of the food system that creates the circumstances leading to contact between food and sick employees.
4. Food Industry Structure and the Magnification of Foodborne Illness
An important feature underlying narrow food safety regulation is its focus on individual farms, food processors, and food products. Many of the examples discussed earlier in Part II.C reflect this pattern, wherein food safety regulation looks only at the moment of contamination of particular food products. Each example above demonstrates that this approach misses underlying causes of narrow food safety risk that stem from intermediate food safety (Part II.C.1) and broad food safety (Parts II.C.2 & 3). In addition, this approach to narrow food safety ignores how the structure and scale of the food system itself might exacerbate foodborne illness risks.
Sociologists Diana Stuart and Michelle Worosz have linked industrialization—in particular “large-scale production, profit-orientation, and technological optimism”—to “widespread outbreaks of foodborne illness.”[325] Stuart and Worosz describe agglomeration practices in the meat processing and leafy greens industries that magnify risks that might otherwise have narrower reaches.[326] In part because both industries rely on processing large quantities of greens or beef from many farms, both expand the potential reach of contamination from an individual farm.[327] For example, a 2011 Listeria outbreak infected 147 people across twenty-eight states and contributed to thirty-three deaths.[328] The outbreak was traced to a single packing facility in Colorado.[329] Similarly, in 2006, an E. coli outbreak that infected 199 people across twenty-six states was traced to one manufacturing facility, and evidence suggested that all the contaminated products were processed in a single day.[330]
The FSMA addresses these consolidation-related risks by emphasizing traceability.[331] Traceability makes it easier to respond to outbreaks, but it has only an indirect role in preventing outbreaks. Because traceability increases the potential of getting caught, it may deter food producers from acting negligently. But traceability does not deal directly with the fact that agglomeration magnifies risk in ways that lie beyond the responsibility of individual players in the food system. Moreover, because the FDA’s Produce Safety and Preventive Controls Rules impose disproportionately higher costs on small-scale farms and food businesses,[332] they may in fact exacerbate consolidation in food production ownership. A USDA assessment of the FDA’s cost benefit analysis concluded that compliance costs will hit small producers the hardest—“very small” farms will face costs up to 6.8 percent of revenue; whereas “large” farms will face compliance costs just below 1 percent.[333] The FSMA created an exemption for farms that receive most of their revenue from sales to “qualified end users,” meaning consumers, restaurants, or retailers within the state or within 275 miles,[334] and it authorized the FDA to provide an exemption for “very small” farms, which the FDA later defined as farms selling less than $25,000 in produce per year.[335] Yet, even eligible farmers may not take advantage of these exemptions because of pressure from buyers and insurers.[336] Consider an analogous situation in the livestock industry. In 2000, small and very small slaughter and processing plants faced implementation of a set of food safety rules similar to those in the FSMA’s Preventive Controls Rule.[337] The increased regulatory burden produced a significant loss of small and mid-sized facilities.[338]
III. Implementing the New Food Safety
As Part II demonstrates, the primacy of narrow food safety in our regulatory system in fact undermines public health. Siloing various food system risks makes it harder to allocate resources and assess tradeoffs. A new, more comprehensive definition of food safety, one that addresses safety across the food system, could mitigate these concerns.
A. Defining Food System Safety
As the first step toward more rational, effective, and efficient food-related health regulation, we call for a reconceptualization of food safety as “Food System Safety.” The risks associated with narrow, intermediate, and broad food safety all stem from the same characteristics of the food system—the ways in which food is produced, distributed, consumed, and thrown away. Because these risks all share root causes, regulating in one area affects the others directly.
Although adopting this definition does not immediately resolve the challenges raised in Part II—unaccounted-for tradeoffs among the traditional silos of food-related health risks and gaps in regulation of food-related health risks—it provides a platform from which resolution might be easier.
The unified definition offers two distinct benefits over the traditional definition. First, a unified definition establishes the appropriate scope for a tradeoff analysis. “Scoping” is essential in the context of affirmative regulatory decisions, e.g., how to set standards and what costs and benefits to include in an economic impact assessment. For instance, a unified definition would invite more robust inquiry into a proposed narrow food safety regulation’s impacts on public health. Scoping is also important in the context of initial decisions about allocation of regulatory resources. For example, a decision about a new narrow food safety regulation would invite conversation about whether new investment in narrow food safety is appropriate in light of under-investment in nutrition. Of course, a broader definition does not ensure that regulators will accurately or fully account for all tradeoffs in individual regulatory decisions, and the definition itself would not guarantee any particular outcome. But, at a minimum, it would set up a hurdle to investment in narrow food safety without consideration of these other aspects of food system health.
Second, a unified definition invites a broader range of participants into regulatory debates. Definitions often control who participates in the decision-making process.[339] Increased participation can improve the quality of decisions by educating decision makers, by educating citizens, and by increasing public buy-in in decisions.[340] For instance, an environmental organization with limited bandwidth to participate in rulemaking processes might be less likely to invest resources in rulemaking addressing narrow food safety than in one addressing Food System Safety. The FSMA rulemaking process, though it had significant environmental implications, drew few environmental commenters in part because of the rule was framed solely as a narrow food safety issue.[341]
Both of these advantages point to an underlying core problem in food regulation: lack of access to information. Information barriers hinder both regulators attempting to develop and implement systemic policies and consumers attempting to choose among individual food products.[342] A broader definition of food safety is a first step toward improving data. It invites more comprehensive analysis and broader participation in decision-making by regulators, and it also invites consumers to consider the interrelated nature of food system issues so that they may demand information that takes those interactions seriously rather than remaining unidimensional.
Currently, detailed data exists only for some categories of food safety. Indeed, in our analysis in Part II we identify a number of areas where our work was hindered by lack of available data. For example, there is no comprehensive data on the costs of food system environmental impacts. Adopting Food System Safety as a regulatory priority could spur the data gathering mechanisms of the federal government—including the GAO and the USDA’s Economic Research Service.[343] It could also lead to NIH and USDA investment in academic research in these areas. Improved data would in turn improve the capacity of federal agencies to assess regulatory decisions within the framework of Food System Safety.
Why persist with the word “safety” rather than rename the problem entirely?[344] Food safety, as we discuss in Part I, has intuitive appeal and conveys urgency. And although policymakers have historically defined the term narrowly, there is at least some evidence that consumers have not always done so. For instance, an early 1980s Food Marketing Institute (FMI) survey found that “[a]lthough pollsters and food professionals distinguish between nutritional content and food safety, the public doesn’t.”[345] A more recent study, a collaboration between Deloitte, FMI, and the Grocery Manufacturers Association, showed that a shift in consumer perspectives “has led to a blurring of lines” between nutrition-related health concerns and safety-related health concerns.[346] The study found that 41 percent of consumers surveyed said they considered “nutritional content” when they “think about safe food and beverages.”[347]
The story is different for broad, as opposed to intermediate, food safety concerns. Consumers typically treat broad food safety concerns as issues of “social impact.”[348] Although consumers, especially younger populations, increasingly identify social impact issues as relevant to their purchasing decisions, social responsibility is significantly less important than personal ingestion-related health.[349] The move to consider environmental protection and workplace safety alongside nutrition and narrow food safety thus requires a shift from the existing dominant consumer perspective.
Beyond the consumer perspective, a growing number of scholars and advocates are calling for a new approach that incorporates consumer health needs with the cradle-to-grave health concerns of Food System Safety.[350] This approach recognizes the various interrelated public health aspects of the entire food system. For instance, the American Public Health Association explains:
In the United States, obesity and diet-related chronic disease rates are escalating, while the public’s health is further threatened by rising antibiotic resistance; chemicals and pathogens contaminating our food, air, soil and water; depletion of natural resources; and climate change. These threats have enormous human, social, and economic costs that are growing, cumulative, and unequally distributed. These issues are all related to food—what we eat and how it is produced.[351]
A new food safety regime offers an opportunity to incorporate this approach into our legal infrastructure. In the following Sections, we offer a variety of paths forward for operationalizing Food System Safety. These proposals outline institutional structures and procedures that might facilitate its adoption, with the ongoing goal of advocating for a new approach to food system health risks that does not strictly silo nutrition, environmental protection, and workplace safety from food safety regulation.
B. Paths to Address Food System Safety
Operationalizing Food System Safety requires making substantive changes to the current risk assessment criteria. In this Section, we first consider the FDA’s own authority and capacity to adopt Food System Safety. Although the agency has broad authority on paper, it does not have a strong track record for robust implementation, even within the realm of narrow food safety.[352] Next, we look at institutional changes that could improve agency decision making with regard to Food System Safety. We explore the possibility of improved coordination and shared goal-setting among federal agencies that are responsible for narrow food safety and those that are responsible for other aspects of Food System Safety. With a broader view of Food System Safety and a method for cross-agency coordination, these agencies can take a more holistic approach to risk regulation. The advantage of this approach is that it allows for attention to Food System Safety without demanding significant change from the agencies focused on narrow food safety. Finally, we consider creation of a unified Food System Safety agency, whose mandate would encompass the full scope of food safety. Although this option perhaps has the greatest potential to achieve the substantive goals set out in this paper, it is also the least politically feasible.[353]
1. FDA Discretion
Although more significant change within the FDA would require statutory amendment, the agency has some leeway to engage in its own reform. Such changes within the FDA could elevate the importance of addressing intermediate risks in tandem with narrow risks. They would be less useful, however, for incorporating consideration of broad food safety risks; for these risks, agency level changes might improve tradeoff analyses but would be unlikely to make significant inroads into resource allocation issues.[354] We conclude that some internal reforms are legally possible, but that the FDA’s history and funding make such an approach challenging.
On paper, the FDCA’s definition of food safety is broad. In the food context, it defines “safe” by “reference to the health of man or animal.”[355] Although a variety of more specific regulatory directives task the FDA with prioritizing ingestion-related safety concerns, and microbial contamination in particular, the FDA could nevertheless use its sweeping rulemaking authority to promulgate a regulatory definition of food safety that incorporated the full range of Food System Safety concerns. Such a definition would not, of course, provide a new source of regulatory authority, but it might shape agency priority setting and analysis within the existing realms of its power.
Overall, the FDA has significant discretion over priority setting and allocation of resources. It could use that discretion to decrease narrow food safety spending, currently about 98 percent of its food budget, and increase nutrition spending, currently about two percent of its food budget.[356] It could also begin to prioritize cumulative ingestion-related risks by setting and evaluating progress towards program measures for its nutrition-related objectives.[357]
The FDA could, within its current authority, use more aggressive regulatory tools to address nutrition. A variety of issues that we identify as areas of nutrition under-regulation—such as animal antibiotics, GRAS additives, and sugar overconsumption—fall within the FDA’s power. After redefining food safety, the agency might be empowered to prioritize these issues and use the command and control regulatory tools typically used for narrow food safety to address them. For instance, it could withdraw or restrict approvals of animal antibiotics used for growth promotion, and begin a robust enforcement program of misuse. It could also consider whether sugar is GRAS, potentially determine that it is not, and trigger a food additives petition for sugar that would allow the agency to cap levels of added sugar.
Within individual agency decisions, the FDA might also take advantage of its obligations to engage in cost benefit and environmental impact analyses to better manage tradeoffs between narrow food safety and broad food safety. Although our analysis above suggests that the FDA has not historically used these tools in this way, a broader agency-level definition of food safety could serve as a jumping off point for consideration of both ancillary public health costs of approaches to narrow food safety and of potential co-benefits of different narrow food safety strategies. For instance, rather than prioritize sterilization of farm environments, a new food safety definition might result in prioritizing healthy microbial diversity, which can both increase soil fertility and combat microbial contamination of produce.
Although the FDA could do more, its potential to act on its own, even if it had the political will to do so, is limited. The agency has little regulatory authority in some of the key policy arenas—such as CAFO management—that are essential to Food System Safety. Further, lack of resources at the FDA has been a persistent problem, and many have critiqued the agency for its limited enforcement even in areas within the narrow food safety mission.[358] Lastly, the primacy of the agency’s narrow food safety mission, while somewhat flexible, is also deeply imbedded in its statutory mandates, its regulations, and its expertise. Shifting this regulatory momentum may require an external force. The next two sections consider possible external forces.
2. A National Food Strategy
Because Food System Safety does not map directly onto the policy goals of any one federal agency, we consider the possible benefits of interagency coordination.[359] With adequate mechanisms for coordination and clear regulatory priorities, such an approach might serve to encourage individual agencies to regulate with Food System Safety in mind. Creation of a US national food strategy could allow other agencies with expertise and political will to act on other food system safety issues to engage with FDA in decision making.
There are many tools that the President or Congress can use in service of interagency coordination. Scholars Jody Freeman and Jim Rossi catalogue these into four main categories: (1) “interagency consultation”; (2) “interagency agreements”; (3) “joint policymaking”; and (4) “Presidential [m]anagement of [c]oordination,” which includes “councils, task forces, and high-level offices . . . aimed at promoting interagency ‘collaboration.’”[360] A national food strategy could use several of these tools—including interagency consultation, the creation of a White House office, or an interagency council or working group—to redefine food safety and pull together the fragmented regulatory regime. Potential benefits of this approach would be to “reduce administrative redundancy, increase legislative and agency coordination, and improve health, economic, and environmental outcomes.”[361] Implementation of a national food strategy would also allow an opportunity to assess and prioritize where tradeoffs are necessary between competing agency goals or food system priorities.[362]
Several other countries, including the United Kingdom and Brazil, have formed national food strategies to address similar challenges.[363] For instance, the UK’s national food strategy, Food 2030, responds to calls “for better integration of food policy across [the UK’s] Government” and for the need to address “the big food challenges—sustainability, security, and health.”[364] The strategy stresses the importance of the commitment to continuous improvements in narrow food safety, but views it in balance with a range of goals, including addressing diet-related disease, ensuring consumer access to healthy and affordable foods, reducing waste, and increasing sustainability by better managing impacts on the ecosystem.[365]
The United States has used national strategies to address a multitude of other complex issues. These national strategies often rely on an organizing entity, such as a single office in the executive branch, an interagency working group, or some combination of the two.[366] Often, these strategies include input from experts in the form of advisory councils. One example is the National HIV/AIDS President’s Advisory Council, “which consist[ed] of diverse members[], including activists and doctors,” and provided input on the development of the National HIV/AIDS Strategy.[367] Such a cross-agency structure could be particularly beneficial in the Food System Safety context, as an interagency working group can bring together relevant expertise to ensure that agency actions properly account for any tradeoffs. It can also help chart a course for policies that provide for the greatest risk reduction, rather than achieving a reduction in narrow food safety at the expense of greater risk in intermediate or broad food safety.
In the context of narrow food safety, the US has taken small steps toward interagency coordination. In 2009, President Obama launched the Food Safety Working Group, which attempted to improve coordination between the USDA and the FDA.[368] The group stopped meeting in 2011 after concluding there were “other collaborative mechanisms” in place that negated the need for additional meetings.[369] Yet, according to a GAO report on high-risk areas of US government operations, these “existing mechanisms” for promoting regulatory coordination fail to provide opportunities for “broad-based, centralized collaboration” between agencies in order to formulate long-term food safety goals and a performance plan to reach those goals.[370] No further attempts have been made to coordinate or address tradeoffs across the food system.
Over the past four decades, GAO flagged the issue of fragmentation of the US food safety system, including at times calling for a single food safety agency[371] or recommending the creation of a government-wide food safety performance plan.[372] Most recently, in 2017, GAO began calling for a national food safety strategy to address the challenges of fragmentation.[373] It noted, “complex interagency and intergovernmental efforts, which could include food safety, can benefit from developing a national strategy and establishing a focal point with sufficient time, responsibility, authority and resources to lead the effort.”[374]
Although the GAO analysis focuses only on narrow food safety, the creation of such a strategy also presents an opportunity to coordinate regulation of the full scope of Food System Safety. GAO is not the first to call for a national food strategy, and other commentators have conceptualized the scope of the strategy even more broadly.[375] Calls for a more coordinated and strategic approach to US food system priorities increased markedly in the lead up to the 2016 election.[376]
A variety of tools for interagency coordination might improve efficiency and efficacy of federal regulation of food safety. Regardless of the mechanism for coordination, however, the effort should focus not on narrow food safety or on any other isolated food system risk. The effort must begin with a comprehensive definition of food safety. Even absent broader congressional directives for regulation of nutrition, environmental protection, and workplace safety, such a coordination effort could, at a minimum, ensure that efforts to improve narrow food safety were not counterproductive and did not exacerbate these other categories of risk.
3. A Unified Food System Safety Agency
Reorganization of federal food agencies has long been a popular topic within the food law community.[377] As noted earlier, fifteen federal agencies share responsibility for administering at least thirty food-related laws.[378] Often, agency authority to regulate food safety is overlapping and confusing. Both the FDA and EPA, for example, have some authority over pesticide use.[379] The FDA has authority to regulate antibiotic use in raising livestock,[380] yet the USDA determines whether meat producers can claim “no antibiotics added” on product labeling.[381] Another commonly-cited example of this overlap is frozen pizza: the FDA regulates frozen cheese pizza, but the USDA regulates frozen pepperoni pizza.[382] Distributing authority in this way interferes with the opportunity for an effective, holistic assessment of risk across the system because at present no single agency can regulate the entire lifecycle of a food product through production, processing, distribution, and labeling, to ultimate consumption and disposal.
Because so many agencies play a role in ensuring the safety of food throughout its lifecycle, GAO includes fragmented federal oversight of food safety in its “High Risk List” of “agencies and program areas that are high risk due to their vulnerabilities to fraud, waste, abuse, and mismanagement, or are most in need of transformation.”[383] Many have proposed consolidation into a single agency.[384] In 2015, Representative Rosa DeLauro and Senator Dick Durbin introduced the Safe Food Act, which would have created a new executive-level food safety agency combining many of the same functions.[385] President Obama’s fiscal year 2016 budget proposal called for consolidation of the food safety-related USDA and FDA functions into a single agency that would sit within the Department of Health and Human Services.[386] In 2018, President Trump proposed to consolidate food safety functions into a single “Federal Food Safety Agency” within the USDA.[387]
A consolidated agency can serve to prioritize a salient issue of national importance. Two prominent examples demonstrate this point. First, in 1970, President Nixon proposed and Congress approved a plan to create the EPA to improve upon the previously piecemeal approach to environmental protection.[388] Indeed, Congress made an explicit decision to keep environmental functions grouped on their own, separate from natural resource extraction oversight functions.[389] Lawmakers were concerned that an agency combining environmental protection and resource extraction would dilute environmental interests by requiring regulators to balance those interests with resource interests.[390] Second, as a result of the September 11, 2001 attacks, Congress formed the Department of Homeland Security by drawing together components of several preexisting agencies into “a new mega-agency.”[391] In both cases, the goal of consolidation was to ensure that a single priority issue had a regulatory champion who was able to examine all aspects of the issue without the burden of attempting to engage in interagency coordination.
Reorganization of food safety into a single agency along the lines of existing proposals could magnify the emphasis on narrow food safety by further entrenching the same narrow food safety focus and priorities in a new food safety agency. As with existing efforts to improve cross-agency coordination, calls for consolidation of agency functions have focused almost exclusively on narrow food safety.[392] Consolidation of only narrow food safety functions, instead of all Food System Safety functions, would further elevate narrow food safety and could exacerbate the problems described in Part II. A single narrow food safety agency would give that issue greater precedence over other food system safety issues that do not have such strong regulatory centers, and would limit opportunities to balance the risks endemic to the food system.
Even with a Food System Safety mandate, a single agency might continue to prioritize narrow food safety because of widely-held beliefs about what constitutes “food safety.” Under its current framework, the FDA prioritizes narrow food safety even where it has the discretion to incorporate other considerations such as nutrition. Results from narrow food safety regulation are relatively easy to measure and highly salient to both consumers and regulated industry.[393] Thus, consolidation alone will not resolve the challenges we have identified. Instead, consolidation of functions must be accompanied by an express and enforceable directive to give adequate attention to risks beyond narrow food safety and adequate resources to address the full scope of food system risk.
A single agency with a mandate that extends beyond narrow food safety could fill existing regulatory gaps relating to many aspects of nutrition, environmental protection, and workplace safety.[394] Further, providing a single agency with consolidated authority over Food System Safety would be responsive to the concern identified in Part II: namely, the interrelated nature of many food system health issues.[395] A single agency, given a food systemwide mandate, could coordinate multiple regulatory goals and balance tradeoffs among them.
Agency unification, however, is a costly and challenging prospect.[396] In 1989, the GAO estimated the costs of agency consolidation at between $447 and $477 million.[397] And, as Freeman and Rossi noted in their analysis of overlapping agency activity, “consolidation cannot be the answer to all of the problems posed by agencies’ sharing regulatory space.”[398] As they explained, “the choice of organizational form . . . may be less important for effectiveness than are coordination and information sharing.”[399]
Thus, although much discussed, a significant overhaul of the food safety regulatory structure is unlikely. Because of the costs involved and the risks of further entrenching the narrow definition of food safety, a single food agency is both unlikely to occur and uncertain to succeed. Consolidation was first proposed in the 1970s, and despite revisiting food safety several times, Congress has never taken it up seriously.[400] Yet, consolidation of food safety regulation into a single Food System Safety agency, if done as we envision, could present an opportunity to reconceive of food safety, to reallocate broader food system regulatory responsibilities, and to reprioritize food safety regulatory spending.
Conclusion
The food system affects public health in many interrelated ways, but food system risk regulation is highly fragmented not just among numerous agencies but also into distinct policy silos. A new definition of Food System Safety would break down the policy silos of traditional food safety, nutrition, environmental protection, and workplace safety. It could also provide a framework for coordination or consolidation of fragmented agency authority.
Currently, narrow food safety regulation receives disproportionate attention measured by allocation of resources and by stringency of regulation. And current approaches to regulation of narrow food safety undermine nutrition, environmental protection, and workplace safety, and fail to account for opportunities for synergies among the categories in ways that are self-defeating.
A unified approach to narrow, intermediate, and broad food safety could facilitate the efficiency and effectiveness of food system risk regulation. Food System Safety provides a platform for more rational allocation of resources and evaluation of tradeoffs among competing priorities. It also opens the door for broader participation in priority setting and for production of information that could support improved tradeoff analysis. Whether it is implemented within the existing structure of federal agencies, by some type of interagency task force, or through a more systemic reorganization of food safety regulatory functions into a single Food System Safety agency, such a reorientation of food regulation is worthwhile, as it is essential to the health and function of the food system.
DOI: https://doi.org/10.15779/Z38QR4NR0D.
Copyright © 2019 California Law Review, Inc. California Law Review, Inc. (CLR) is a California nonprofit corporation. CLR and the authors are solely responsible for the content of their publications.
Emily M. Broad Leib is an Assistant Clinical Professor and Director of the Food Law and Policy Clinic at Harvard Law School. Margot J. Pollans is an Associate Professor and Faculty Director of the Pace-NRDC Food Law Initiative at the Elisabeth Haub School of Law at Pace University. We would like to thank Hope Babcock, Oren Bar-Gill, Noa Ben-Asher, Marie Boyd, Bill Buzbee, Bridget Crawford, Jacob Gersen, Blake Hudson, Peter Barton Hutt, Katrina Kuh, Peter Lehner, Timothy Lytton, Lee Miller, and Chip Murphy for helpful comments. Thanks also to our excellent research assistants Lisa Gluckstein, Molly Malavey, Michael McConnell, Sarah Paige, Aaron Rudyan, Emma Scott, and Gabriel Wildgen, and to the tireless work of the editing team at the California Law Review.
- See Renée Johnson, Cong. Research Serv., RS22600, The Federal Food Safety System: A Primer 1 (2016) (noting that “[f]ederal responsibility for food safety rests primarily with the [FDA] and the [USDA]”). The USDA oversees food safety primarily through its Food Safety and Inspection Service (FSIS), which regulates safety of meat, poultry, and unshelled eggs and egg products. Id. at 5–6 (noting that the FDA has jurisdiction over shelled eggs). The “bifurcated system” of food safety dates back to the early 1900s, when Congress divided jurisdiction between the FDA (then the USDA’s Bureau of Chemistry) and the USDA (then the USDA’s Bureau of Animal Industry). Id. at 2. ↑
- See id. at 1. The FDA oversees approximately 80 percent of the US food supply, including the labeling of most domestic and imported foods other than meat and poultry. Id. at 4 (noting that the “FDA has primary responsibility for the safety of most (about 80%–90%) of all US domestic and imported foods”). The FDA is the “oldest comprehensive consumer protection agency in the US federal government.” The History of FDA’s Fight for Consumer Protection & Public Health, U.S. Food & Drug Admin., https://www.fda.gov/AboutFDA/History/default.htm [https://perma.cc/NJ83-XH7M]. ↑
- See, e.g., Richard Zeckhauser, Measuring Risks and Benefits of Food Safety Decisions, 38 Vand. L. Rev. 539, 557–70 (1985) (describing some of the challenges of developing regulations that balance risks and benefits efficiently in the context of food additives). ↑
- Although there is a robust literature in each of the traditional policy areas that make up food-related health—food safety, nutrition, environmental protection, and worker safety—the legal literature examining the interaction of food safety with other food-related health issues is limited. Only a handful of legal scholars have begun to call for this approach. See, e.g., Laurie J. Beyranevand & Emily M. Broad Leib, Making the Case for a National Food Strategy in the United States, 72 Food & Drug L.J. 225, 226–29 (2017) (explaining that several food law and policy articles “focus on discrete food system issues, such as food safety” and arguing that there are a variety of challenges associated with an “uncoordinated approach” to food); Bruce Friedrich & Stefanie Wilson, Coming Home to Roost: How the Chicken Industry Hurts Chickens, Humans, and the Environment, 22 Animal L. 103, 119–28, 143–57 (2015) (examining the range of human health and environmental effects of modern chicken production); Margaret Sova McCabe, Foodshed Foundations: Law’s Role in Shaping Our Food System’s Future, 22 Fordham Envtl. L. Rev. 563, 589 (2011) (introducing a foodshed model and “invit[ing] readers to imagine how a foodshed might help us realize a sustainable, efficient, and healthful food system”); Margot J. Pollans, Regulating Farming: Balancing Food Safety and Environmental Protection in a Cooperative Governance Regime, 50 Wake Forest L. Rev. 399 (2015) [hereinafter Pollans, Regulating Farming] (examining tradeoffs between food safety and environmental protection in the new Food Safety Modernization Act (FSMA) regime); Gabriela Steier, Dead People Don’t Eat: Food Governmentenomics and Conflicts-of-Interest in the USDA and FDA, 7 Pitt. J. Envtl. Pub. Health L. 1, 50 (2012) (arguing that fragmentation of food regulatory authority makes it easier for “Big Food” to achieve regulatory capture). Beyond the legal literature, there is a growing debate about the need for a systems approach to understanding and regulating food-related public health. See infra Part III.A (discussing calls for a systems approach). ↑
- For discussion of agency myopia, see Jeffrey J. Rachlinski & Cynthia R. Farina, Cognitive Psychology and Optimal Government Design, 87 Cornell L. Rev. 549, 580 (2002) (explaining how “agencies can become myopically focused on their missions”); Samuel J. Rascoff & Richard L. Revesz, The Biases of Risk Tradeoff Analysis: Towards Parity in Environmental and Health-and-Safety Regulation, 69 U. Chi. L. Rev. 1763, 1766 (2002) (observing that when risk tradeoff analysis occurs, it “systematically ignores the phenomenon of ‘ancillary benefits,’ reductions in risk that take place in addition to—and as a direct or indirect result of—reductions in the target risk”). ↑
- This division of food system health risks parallels the problems of agencies separating a larger project into smaller steps during environmental impact assessments. If a project is too subdivided, it may appear to have a smaller impact than it actually does. 40 C.F.R. § 1508.27(b)(7) (2018) (“Significance cannot be avoided by . . . breaking [an action] down into small component parts.”). The same problem exists in cost-benefit analysis. Changes in scope of analysis can lead to wildly disparate results. See, e.g., Rascoff & Revesz, supra note 5, at 1763 (observing that cost-benefit analysis tends to exclude analysis of “ancillary benefits”). ↑
- “Throughout its history FDA has had essentially the same assignment: to assure that the products it regulates are safe and truthfully labeled.” Peter Barton Hutt et al., Food and Drug Law: Cases and Materials 4 (4th ed. 2014). ↑
- We contend that the definition of food safety matters. Without redefinition, we might preserve existing policy silos and ensure that intermediate and broad food safety risks are better addressed by giving them more robust attention elsewhere. But such a solution, though perhaps an improvement on the status quo, would be undesirable. The vocabulary used to define food safety controls the scope of the conversation not only by prioritizing certain kinds of risks over others, but also by predetermining which federal agencies play primary roles. Problem definition signals who should participate in the conversation, controls who is held accountable and for what, and shapes what kinds of data is systematically collected and analyzed. See Janet A. Weiss, The Powers of Problem Definition: The Case of Government Paperwork, 22 Pol’y Sci. 97, 99 (1989) (describing how the definition of a policy problem can have significant consequences for the rest of the policy process, from “policy deliberation,” “political debate,” and “the ultimate products of policy action”); see also Eric Biber, Too Many Things to Do: How to Deal with the Dysfunctions of Multiple-Goal Agencies, 33 Harv. Envtl. L. Rev. 1, 61–62 (2009) (observing that complications arise when a single agency has multiple directives; for a variety of practical reasons, “multiple-goal agencies” will likely prioritize one goal over the others). ↑
- Richard B. Stewart, A New Generation of Environmental Regulation?, 29 Cap. U. L. Rev. 21, 35–36 (2001) (using the phrase “tunnel vision” to describe the phenomenon of agencies becoming “insensitiv[e] to the broader range of interests, values, and considerations at stake in their decisions” when agencies are “driven by their organizational missions and the interests of their organized client constituencies”). Here, agency “tunnel vision” arises from the fact that Congress has assigned it a single mission, or a single primary mission. ↑
- In making these suggestions, we draw from the literature on allocation of regulatory responsibilities among federal agencies. See, e.g., Jody Freeman & Jim Rossi, Agency Coordination in Shared Regulatory Space, 125 Harv. L. Rev. 1131, 1134–35 (2012) (explaining why Congress often give agencies overlapping regulatory responsibilities); Anne Joseph O’Connell, The Architecture of Smart Intelligence: Structuring and Overseeing Agencies in the Post-9/11 World, 94 Calif. L. Rev. 1655, 1657 (2006) (arguing that unifying agency activities and oversight may undermine policy goals). ↑
- Marion Nestle, The Law and Nutrition, 66 N.Y. St. B.J. 38, 40 (1994) (noting that “food safety regulation” and “nutrition services in health care reform” are “especially likely to stimulate future legislative activity: food safety regulation”). As a counter example, nutrition scholar Joan Dye Gussow defines food safety with regard to the “‘bads’ all of us seek to avoid. We look at the safety of the food supply and at threats to that safety that some people believe may come either from the activities of food technologists or from the contamination of the food producing environment by farmers or industry . . . .” Joan Dye Gussow & Paul R. Thomas, The Nutrition Debate: Sorting Out Some Answers 343 (1986); see also Chris Lecos, Pesticides and Food: Public Worry No. 1, 18 FDA Consumer 12 (1984), reprinted in id. at 388 (explaining that “pollsters and food professionals distinguish between nutritional content and food safety, [but] the public doesn’t”). ↑
- What We Do, U.S. Food & Drug Admin., https://www.fda.gov/AboutFDA/WhatWeDo/default.htm [https://perma.cc/PE2L-6KXF]. ↑
- Safety, Merriam-Webster Dictionary. Synonyms include protection, safeness, and security. ↑
- 21 U.S.C. § 321(u) (2012). This definition applies to food additives (§ 321(s) and § 348), new animal drugs (§ 360(b) and § 360(c)), and color additives (§ 379(e)). ↑
- 21 U.S.C. § 331(a) (prohibiting sale of “misbranded” products); see also id. § 371(a) (establishing FDA’s enforcement authority). Although there are some safety-related functions of the FDA’s regulations related to misbranding (for instance, authority to implement misbranding regulations to address allergens in § 343(w)), most misbranding regulations are related to issues of fraud or economic adulteration. See id. § 343(a) (defining food misbranding primarily as “false or misleading” labeling). Thus, the bulk of the FDA’s food safety functions are linked to its authority over adulteration. ↑
- Id. § 342(a)(1) (providing that food is “adulterated” “[i]f it bears or contains any poisonous or deleterious substance which may render it injurious to health”). This statutory definition of “adulterated” distinguishes between added and inherent substances, setting a higher threshold for adulteration if the substance is not an added substance. Id. ↑
- See U.S. Food & Drug Admin., Defect Levels Handbook, (May 1995, rev. May 1998), https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/SanitationTransportation/ucm056174.htm [https://perma.cc/AYP5-ABLE]. For instance, if one hundred grams of apple butter contains more than 12 percent mold, five whole insects, or four rodent hairs, it is considered adulterated. Id. ↑
- See 21 U.S.C. § 342(a)(1) (defining adulteration as food that “consists . . . of any filthy, putrid, or decomposed substance”); 21 C.F.R. § 110.110 (2018) (establishing FDA authority to set tolerance levels for contaminants that pose no health risk). The FDA has only promulgated a binding tolerance level once, and instead typically uses “non-binding action levels.” Marie C. Boyd, Cricket Soup: A Critical Examination of the Regulation of Insects as Food, 36 Yale L. & Pol’y Rev. 17, 53 n.187 (2017). ↑
- 21 U.S.C. § 342(a)(4). The FDA relies on the authority of 21 U.S.C. § 342(a)(4) to promulgate regulations governing sanitary conditions for certain foods with high risk for microbial contamination. See, e.g., 21 C.F.R. § 120.9 (2018) (establishing that failure to comply with juice regulations “render[s] the juice products . . . adulterated under [21 U.S.C. § 342(a)(4)]); Hazard Analysis and Critical Control Point (HAACP), Procedures for the Safety and Sanitary Processing and Importing of Juice, 66 Fed. Reg. 6137, 6158 (Jan. 19, 2001) (final rule) (codified at 21 C.F.R. pt. 120) (explaining the agency’s statutory justification for promulgating the regulation to create sanitary procedures for juice processing, because without such procedures, “it is reasonably possible that the juice may be rendered injurious to health” and thus be adulterated). ↑
- Hutt et al., supra note 7, at 528 (noting that this provision is “the most important pre-FSMA statutory provision for addressing pathogenic contamination of food”). ↑
- 21 U.S.C. § 350c(a)(2). ↑
- See 21 U.S.C. § 350g (establishing requirements for preventive controls at food processing facilities); id. § 350h (establishing standards for produce safety in agricultural growing, harvesting, and packing). The FDA always had authority to impose liability on farms responsible for food safety outbreaks, but the FSMA expressly requires the agency to develop preventive standards for on-farm practices. Some commentators have argued that given the broad language of the FDCA, the agency always had this authority. See Peter Barton Hutt, Philosophy of the Regulation Under the Federal Food, Drug and Cosmetic Act, 50 Food & Drug L.J. 101, 102 (1995) (“Except where expressly prohibited, I believe the Food and Drug Administration is obligated to develop whatever innovative and creative regulatory programs are reasonable and are most appropriate to achieve the fundamental objectives laid down by Congress.”). The FSMA is designed to make the FDA’s regulatory work preventive rather than responsive. See Pollans, Regulating Farming, supra note 4, at 413–17 (describing FSMA’s history). ↑
- 21 U.S.C. § 350h(a)(3)(B) (directing the FDA to promulgate rules that establish “with respect to growing, harvesting, sorting, packing, and storage operations, science-based minimum standards related to soil amendments, hygiene, packaging, temperature controls, animals in the growing area, and water”) and § 350h(a)(1)(A) (directing the FDA to publish a notice of proposed rulemaking within one year of enactment of the Act). ↑
- See 21 C.F.R. pt. 112. ↑
- Id. The FDA promulgated this rule under the FSMA, which required the FDA to conduct a rulemaking to “establish science-based minimum standards for the safe production and harvesting of those types of fruits and vegetables . . . for which the Secretary has determined that such standards minimize the risk of serious adverse health consequences or death.” 21 U.S.C. § 350h(a)(1)(A). ↑
- 21 U.S.C. § 350h(a)(3)(B) (identifying these six areas for regulation); Standards for Growing, Harvesting, Packing, and Holding of Produce for Human Consumption, 80 Fed. Reg. 74,354, 74,356 (Nov. 27, 2015) (to be codified at 21 C.F.R. pt 11,16,112) (summarizing the main components of key provisions of the rule). ↑
- 21 C.F.R. § 112.129 (describing toilet facility requirements); id. § 112.130 (describing hand-washing facility requirements). ↑
- Id. § 112.127(a) (describing requirements regarding domesticated animals in and around enclosed buildings). ↑
- See 21 C.F.R. pt. 117; Current Good Manufacturing Practice and Hazard Analysis and Risk-Based Preventive Controls for Human Food, 79 Fed. Reg. 58,524 (Sept. 29, 2014) (supplemental notice of proposed rule). ↑
- See World Health Org. & Food & Agric. Org., Diet, nutrition and the prevention of chronic diseases 1–2 (2003). ↑
- See, e.g., Hutt et al., supra note 7, at 489 (introducing the “regulation of the safety of food constituents”); Marion Nestle, Safe Food: The Politics of Food Safety 1–2 (2010) (focusing discussion of food safety around “foodborne illness, food biotechnology, and food bioterrorism”). Because it receives less regulatory attention, we focus, for the most part on narrow food safety only in our analysis in Parts II and III. There are a few instances, however, where we consider traditional food safety regulation as a whole, including both narrow food safety and the food additives functions of intermediate food safety. ↑
- See 21 U.S.C. § 348. ↑
- Id. § 321(s). ↑
- Id. § 348(a)(2). Although the FDA regulates most food additives, the EPA has authority over pesticide residues; it can set maximum residue levels of a pesticide on food and animal feed. 40 C.F.R. § 180.3 (2018); U.S. Food & Drug Admin., Pesticide Residue Monitoring Program Fiscal Year 2016 Pesticide Report 8 (2018), https://www.fda.gov/media/117088/download [https://perma.cc/24TF-YQSG]. If EPA has not set a tolerance level, and an exemption does not apply, FDA can issue a nonbinding “action level” for unavoidable residues. Id. ↑
- 21 U.S.C. § 348(c)(3)(a) (establishing that “no additive shall be deemed to be safe if it is found to induce cancer when ingested by man or animal”). ↑
- Id. § 321(s). ↑
- Substances Generally Recognized as Safe, 81 Fed. Reg. 54,960 (Aug. 17, 2016) (to be codified at 21 C.F.R. pts. 20, 25, 170, 184, 186, 570); Substances Generally Recognized as Safe, 62 Fed. Reg. 18,938 (Apr. 17, 1997) (proposed rule). The final rule replaces a voluntary “affirmation process” with a voluntary “notification procedure,” but the procedure retains the core similarity that manufacturer’s duty to alert the FDA of the conclusion that a substance is GRAS for its intended use is voluntary in nature. The final rule does not substantially differ from the FDA’s older procedure, which was also voluntary but allowed companies to ask the FDA for regulation declaring a substance GRAS. See About the GRAS Notification Program, U.S. Food & Drug Admin., (Oct. 2016) https://www.fda.gov/food/ingredientspackaginglabeling/gras/ucm2006851.htm [https://perma.cc/XF9H-9YGX]; FDA’s Approach to the GRAS Provision: A History of Processes, U.S. Food & Drug Admin., https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/ucm094040.htm [https://perma.cc/VRU3-E3E4]. ↑
- See Tom Nelter & Maricel Maffini, Nat. Res. Def. Council, Generally Recognized as Secret: Chemicals Added to Food in the United States 2 (Apr. 2014), https://www.nrdc.org/sites/default/files/safety-loophole-for-chemicals-in-food-report.pdf [https://perma.cc/ZL2X-TKHE]. ↑
- Pew Charitable Trusts, Fixing the Oversight of Chemicals Added to Our Food: Findings and Recommendations of Pew’s Assessment of the U.S. Food Additives Program 5 (Nov. 2013), https://www.pewtrusts.org/-/media/legacy/uploadedfiles/phg/content_level_pages/reports/foodadditivescapstonereportpdf.pdf [https://perma.cc/6D6R-7Q9R]. ↑
- For example, the FDA recently determined that after decades of common use in the food supply, partially hydrogenated oils (PHOs), the majority dietary source of artificial trans fact, were unsafe and, therefore, no longer GRAS. See infra notes 45–50 and accompanying text for description of regulatory history of the PHO ban. ↑
- Complaint for Declaratory and Injunctive Relief at 28–30, Ctr. for Food Safety et al. v. Price, 2018 WL 4356730 (S.D.N.Y. 2018) (No. 17-CV-3833). ↑
- See Adult Obesity Causes & Consequences, Ctrs. for Disease Control & Prevention, https://www.cdc.gov/obesity/adult/causes.html [https://perma.cc/RZ36-TMNA]; see also Jiaquan Xu et al., 67 National Vital Statistics Reports, Deaths: Final Data For 2016, at 1 (July 26, 2016), https://www.cdc.gov/nchs/data/nvsr/nvsr67/nvsr67_05.pdf [https://perma.cc/EZR8-2F9M] (including diabetes, heart disease, hypertension, and stroke among top causes of death in 2016). ↑
- See infra Part II.A for comparison of relative resources dedicated to traditional food safety versus diet related disease. ↑
- See supra Part I.A.1 (describing FDA regulation of narrow food safety). ↑
- In 1994, two public health experts estimated that trans fat in the food supply caused more than 30,000 deaths annually. Complaint ¶ 21, Ctr. for Sci. in the Pub. Interest v. Burger King, 534 F. Supp. 2d 141 (Sup. Ct. D.C. May 16, 2007) (No. 1:07CV01092) (citing a Harvard School of Public Health Report that found that eliminating trans fats could prevent between 30,000 to 100,000 deaths); Walter C. Willett & Alberto Ascherio, Trans Fatty Acids: Are the Effects Only Marginal?, 85 Am. J. Pub. H. 722, 723 (1994). ↑
- Dariush Mozaffarian et al., Trans Fatty Acids and Cardiovascular Disease, 354 New Eng. J. Med. 1601, 1611 (2006). ↑
- Citizen petitions for FDA to remove PHOs from the GRAS list were filed in 2004 and 2009. See Michael F. Jacobson, Ctr. for Science in the Pub. Interests, No. FDA–2004–P–0279, Citizen Petition for Rulemaking to Revoke the Authority for Industry to Use Partially Hydrogenated Vegetable Oils in Foods (May 18, 2004); https://cspinet.org/sites/default/files/attachment/trans_fat_petition_may_18.pdf. [https://perma.cc/S6Z7-HH6C]; Fred A. Kummerow, No. FDA–2009–P–0382, Citizen Petition to Ban Partially Hydrogenated Fat from the American Diet (Aug. 4, 2009), http://archive.wphna.org/wp-content/uploads/2014/01/09-08-FDA-Kummerow-FDA-petition-Hydrogenation.pdf [https://perma.cc/GV3C-XWBD]. It took FDA until 2015 to release its final determination that such substance is not GRAS, and the determination went into effect in June 2018. 80 Fed. Reg. 34,650. ↑
- See Complaint for Declaratory and Injunctive Relief, Kummerow v. U.S. Food & Drug Admin., No. 2:13-cv-02180-HAB-DGB (C.D. Ill. Aug. 9, 2013). ↑
- Final Determination Regarding Partially Hydrogenated Fats, 80 Fed. Reg. 34,650 (June 17, 2015). The FDA had previously imposed a requirement that manufacturers separately list trans fat as part of the standard nutrition label. See 21 C.F.R. § 101.9(c)(2)(ii) (2016); Food Labeling: Trans Fatty Acids in Nutrition Labeling, Nutrient Content Claims, and Health Claims, 68 Fed. Reg. 41,434 (July 11, 2003) (final rule) (establishing effective date of Jan. 1, 2006). ↑
- Final Determination Regarding Partially Hydrogenated Fats, 83 Fed. Reg. 23,358, 23,359 (May 21, 2018) (providing a one-year extension for some uses and an 18-month extension for other uses). ↑
- See FDA Issues Voluntary Sodium Reduction Targets, Ctr. for Science Pub. Interest (June 1, 2016), https://cspinet.org/news/fda-issues-voluntary-sodium-reduction-targets-20160601 [https://perma.cc/T9BF-28EB]. ↑
- U.S. Food & Drug Admin, FDA-2014-D-0055, Draft Guidance for Industry: Voluntary Sodium Reduction Goals: Target Mean and Upper Bound Concentrations for Sodium in Commercially Processed, Packaged, and Prepared Foods (2016), http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ucm494732.htm [https://perma.cc/UZZ4-NMJJ]. ↑
- 21 U.S.C. § 348(c)(4) (2012) (authorizing the FDA to set a tolerance limitation if necessary for safe use of an additive). ↑
- See Lisa Heinzerling, Food Law: Cases & Materials 7–8 (2015) (dividing food law goals into three categories: safety-promoting, knowledge-promoting, and security-promoting). ↑
- See Nutrition Labeling and Education Act of 1990, Pub. L. 101-535, 21 U.S.C. § 343 (2012) (establishing FDA’s authority to regulate misbranding); id. § 343(q) (giving the FDA authority to regulate nutrition information in particular). The FDCA replaced the Pure Food and Drug Act, which also authorized misbranding regulation. Milestones in U.S. Food and Drug Law History, U.S. Food & Drug Admin., https://www.fda.gov/about-fda/fdas-evolving-regulatory-powers/milestones-us-food-and-drug-law-history [https://perma.cc/X4GR-5WXT]. ↑
- 21 U.S.C. § 343(q); Virginia Wilkening, The Nutrition Labeling and Education Act of 1990, U.S. Food & Drug Admin., https://www.nutrientdataconf.org/PastConf/NDBC17/8-2_Wilkening.pdf [https://perma.cc/2GLE-4XED] (characterizing the act as requiring the FDA to engage in an effort “to provide a food label that the public can count on and to upgrade the label to reflect current nutritional science and public health concerns”). The Patient Protection and Affordable Care Act (ACA) of 2010 included a provision requiring nutrition disclosure information on chain restaurant menus. ACA § 4205, 21 U.S.C. § 343(q)(5)(H) (2012). ↑
- See, e.g., § 343(q)(1)(E) (explaining that nutrition information labeling provisions emphasize a regulatory goal of helping consumers “maintain[] healthy dietary practices”). ↑
- Food Labeling: Revision of the Nutrition and Supplemental Facts Labels, 81 Fed. Reg. 33,742 (May 27, 2016) (final rule) (revising nutrition facts panel requirements); Changes to the Nutrition Facts Label, U.S. Food & Drug Admin., http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm385663.htm [https://perma.cc/U52X-HKUP]. In May 2018, the FDA published a final rule delaying implementation of this rule from its original compliance deadline of July 2018 to January 2020 for manufacturers with $10 million or more in annual sales, and from July 2019 to January 2021 for manufacturers with less than $10 million in annual sales. Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of Foods, 83 Fed. Reg. 19,619 (May 4, 2018) (final rule) (to be codified at 21 C.F.R. § 101). ↑
- In 1999, the CSPI petitioned the FDA to require added sugars on the nutrition facts label. Michael F. Jacobson, Ctr. for Science in the Pub. Interests, Citizen Petition to U.S. Food & Drug Admin., U.S. Dep’t of Health & Human Servs., for Proposed Rulemaking to Establish a Daily Reference Value for “Added Sugars,” (Aug. 3, 1999), https://cspinet.org/sites/default/files/attachment/sugar-petition-1999.pdf [https://perma.cc/AT9A-QJ6X]. ↑
- See, e.g., Bruce Silverglade & Ilene Ringel Heller, Ctr. for Science in the Pub. Interest, Food Labeling Chaos: The Case for Reform Part I-5 (Mar. 2010) https://cspinet.org/sites/default/files/attachment/food_labeling_chaos_report.pdf [https://perma.cc/2JWK-A6RL] (“In general, since 2001, there has been a significant decline in labeling enforcement by the FDA.”); Jennifer L. Pomeranz, A Comprehensive Strategy to Overhaul FDA Authority for Misleading Food Labels, 39 Am. J. L. Med. 617, 619 (2013) (“The FDA does not have the resources to sufficiently address the current state of labeling, nor is there funding allocated to feasibly increase its enforcement power.”). ↑
- See, e.g., Silverglade & Heller, supra note 60, at iv (identifying gaps around regulation of commonly used label terms such as “natural” and “whole wheat,” and noting that “[t]he FDA and the USDA should develop regulations instead of relying only on case-by-case enforcement actions”); Pomeranz, supra note 60, at 618 (“Current food labeling practices include both actual misbranding and permissible but potentially misleading claims about the healthfulness of processed foods. The latter is due to regulations that are too lax or do not reflect the most current science on nutrition.”). ↑
- See National Nutrition Monitoring and Related Research Act of 1990 § 301(a); 7 U.S.C. § 5341(a)(1) (2012) (codifying establishment of dietary guidelines and requiring that they “be promoted by each Federal agency in carrying out any Federal food, nutrition, or health program”); id. § 5302(9) (defining “Secretaries”). ↑
- See Food Service Guidelines Federal Workgroup, U.S. Dep’t of Health & Human Servs., Food Service Guidelines for Federal Facilities 10 (2017), https://www.cdc.gov/obesity/downloads/guidelines_for_federal_concessions_and_vending_operations.pdf [https://perma.cc/42VV-NXXT] (explaining that the food service guidelines for federal facilities are based on the Dietary Guidelines for Americans); see also Dietary Guidelines: Process, Office of Disease Prevention & Health Promotion, https://health.gov/dietaryguidelines/process.asp [https://perma.cc/9C8A-6DHZ] (describing the dietary guidelines development process). ↑
- Nutrition Standards in the National School Lunch and School Breakfast Programs, 77 Fed. Reg. 4088 (Jan. 26, 2012) (final rule) (to be codified at 7 C.F.R. pts. 210, 220) (updating “nutrition standards for the National School Lunch and School Breakfast Programs to align them with the Dietary Guidelines for Americans”); School Meals: Child Nutrition Programs, U.S. Dep’t of Agric., https://www.fns.usda.gov/school-meals/child-nutrition-programs [https://perma.cc/UTU6-BGQ4]. ↑
- See, e.g., Margot J. Pollans, Drinking Water Protection and Agricultural Exceptionalism, 77 Ohio St. L.J. 1195, 1197 (2016) [hereinafter Pollans, Drinking Water Protection] (discussing the high levels of nitrate in the drinking water of Des Moines, Iowa, and explaining the potential health consequences of nitrate contamination). ↑
- See id. at 1209–10 (describing how agricultural pollutants caused an algal bloom in the drinking water source of Toledo, Ohio). ↑
- See Hannah M.M. Connor, The Industrialization of Animal Agriculture: Connecting a Model With Its Impacts on the Environment, in Food, Agriculture, and Environmental Law 65, 82–84 (Mary Jane Angelo et al. eds., 2013). Rural communities near feedlots also suffer from severe mental health consequences related to living with the odors of these farms. Kelley J. Donham et al., Community Health and Socioeconomic Issues Surrounding Concentrated Animal Feeding Operations, 115 Envtl. Health Perspectives 317, 318 (2007). ↑
- See, e.g., Geoffrey M. Calvert et al., Acute Pesticide Poisoning Among Agricultural Workers in the United States, 1998–2005, 51 Am. J. Ind. Med. 883, 896 (2008) (finding that agricultural workers have greater risk of acute pesticide poisoning than non-agricultural workers). ↑
- Ctrs. for Disease Control & Prevention, Nat’l Inst. for Occupational Safety & Health, A Story of Impact: NIOSH Pesticide Poisoning Monitoring Program Protects Farmworkers (Dec. 2011), https://www.cdc.gov/niosh/docs/2012-108/pdfs/2012-108.pdf?id=10.26616/NIOSHPUB2012108 [https://perma.cc/3LQA-DCVG]. ↑
- Health & Environmental Implications of U.S. Meat Consumption & Production, Johns Hopkins Bloomberg School of Public Health, https://www.jhsph.edu/research/centers-and-institutes/johns-hopkins-center-for-a-livable-future/projects/meatless_monday/resources/meat_consumption.html [https://perma.cc/43G6-LB55]. ↑
- Ctrs. for Disease Control & Prevention, Antibiotic Resistance Threats in the United States, 2013, at 11 (2013), https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf [https://perma.cc/56KW-H2YT]. ↑
- Antibiotic Resistance, NARMS–Combating Antibiotic Resistance with Surveillance, Ctrs. for Disease Control & Prevention, https://www.cdc.gov/narms/faq.html [https://perma.cc/S2LU-8Q39]. ↑
- Drug disposal is also an issue of growing concern. Large quantities of prescription drugs end up in waterways because unused drugs are flushed down toilets or otherwise disposed improperly, and because human bodies do not metabolize or absorb drugs fully, drugs enter water through skin, urine, and excrement. See Drugs in the Water, Harv. Health Letter, Harv. Med. Sch. (June 2011), https://www.health.harvard.edu/newsletter_article/drugs-in-the-water [https://perma.cc/B76A-NF3E]. Growing concentrations of prescription drugs can affect aquatic ecosystems. See id. ↑
- See Dana Gunders et al., Nat. Res. Def. Council, Wasted: How America Is Losing Up To 40 Percent Of Its Food From Farm To Fork To Landfill 10 (2d ed. 2017), https://www.nrdc.org/sites/default/files/wasted-2017-report.pdf [https://perma.cc/9LK5-JFYU]. ↑
- For a more detailed discussion of these costs, see infra Part II.B.2.a. ↑
- See Jason J. Czarnezki & Elisa K. Prescott, Environmental and Climate Impacts of Food Production, Processing, Packaging, and Distribution, in Food, Agriculture, and Environmental Law 113, 125 (Mary Jane Angelo et al. eds., 2013) (observing that plastics are the most common packaging material); Chelsea M. Rochman et al., Classify Plastic Waste as Hazardous, 494 Nature 169, 169–70 (2013) (arguing that plastics should instead be classified not as solid waste but as hazardous waste, a classification that would result in application of hazardous waste disposal laws). ↑
- Rochman et al., supra note 76, at 170 (assessing global plastic use and disposal). ↑
- See Czarnezki & Prescott, supra note 76, at 126. ↑
- See, e.g., K.G. Harding et al., Environmental Analysis of Plastic Production Processes: Comparing Petroleum-Based Polypropylene and Polyethylene with Biologically-Based Poly-ß-hydroxybutyric Acid Using Life Cycle Analysis, 130 J. of Biotech. 57, 62 (2007) (identifying resource input and pollution costs associated with oil-based plastic production). ↑
- U.S. Envtl. Prot. Agency, EPA 430-R-18-003, Inventory of U.S. Greenhouse Gas Emissions and Sinks, 1990–2016, at ES-21 (Apr. 12, 2018) (estimating that in 2016 agriculture was responsible for 8.6 percent of US GHG emissions), https://www.epa.gov/sites/production/files/2018-01/documents/2018_complete_report.pdf [https://perma.cc/MU5F-XMYF]. ↑
- Sonja J. Vermeulen et al., Climate Change and Food Systems, 37 Ann. Rev. Envtl. Resources 195, 198 (2012) (discussing how the global food system impacts climate change). ↑
- Arjen Y. Hoekstra & Mesfin M. Mekonnen, The Water Footprint of Humanity, 109 PNAS 3232, 3234 (2012). ↑
- Irrigation & Water Use?, U.S. Dep’t of Agric., Econ. Res. Serv., https://www.ers.usda.gov/topics/farm-practices-management/irrigation-water-use/ [https://perma.cc/R24T-XATT]. ↑
- See Daniel A. Farber, Coping with Uncertainty: Cost-Benefit Analysis, the Precautionary Principle, and Climate Change, 90 Wash. L. Rev. 1659, 1689–710 (2015) (discussing challenges in calculating the social cost of carbon). ↑
- See, e.g., J.B. Ruhl, Farms, Their Environmental Harms, and Environmental Law, 27 Ecology L.Q. 263, 266 (2000) (noting that the environmental consequences of farming “have escaped serious regulatory attention” and describing such exemptions in areas of water quality law, air quality law, and toxic waste management law). ↑
- See Jacob E. Gersen, Margot J. Pollans, & Michael T. Roberts, Food Law: Cases and Materials 781–96 (2019) (mapping environmental regulation of food production); J.B. Ruhl, Agriculture and Ecosystem Services: Paying Farmers to Do the New Right Thing, in Food, Agriculture, and Environmental Law 241 (Mary Jane Angelo et al. eds., 2013) (describing green payment programs); Jason Czarnezki, Margot J. Pollans, & Sarah M. Main, Eco-labelling, in Oxford Handbook on Comparative Environmental Law 996, 999, 1006 (forthcoming 2019) (giving some examples of public “eco-label” programs in the United States). ↑
- See, e.g., Guadalupe T. Luna, An Infinite Distance?: Agricultural Exceptionalism and Agricultural Labor, 1 U. Pa. J. Lab. & Emp. L. 487, 489 (1998) (describing labor law exceptions for agricultural workers). ↑
- 21 U.S.C. § 348(h)(6) (2012) (defining “food contact substance,” for the purpose of the food additives regulations, as “any substance intended for use as a component of materials used in manufacturing, packing, packaging, transporting, or holding food if such use is not intended to have any technical effect in such food”). ↑
- Id. §§ 348(a)(3), (h)(1). ↑
- Id. § 348(h)(1); id. § 348(h)(2)(A) (giving the FDA 120 days to reject such a notification and issue a determination that the “food contact substance” is not safe). ↑
- Id. § 348(a)(3) (establishing FDA authority to regulate food contact substances as food additives); see also Margot J. Pollans, FDA and the Environment (Dec. 21, 2018) (unpublished manuscript) (on file with authors) [hereinafter Pollans, FDA and the Environment]. ↑
- See Gersen, Pollans, & Roberts, supra note 86, at Ch. 7 Part II.B (offering a variety of examples of underenforcement of environmental laws as applied to agriculture). ↑
- Behavioral economics arose to supplement and broaden the field of law and economics. See Edwin E. Witte, Economics and Public Policy, 47 Am. Econ. Rev. 1, 2 (1957) (noting that “[m]any of the major advances in economic theory have resulted almost directly from attempts to find solutions for practical public policy questions”). The driving assumption behind the law and economics field is that human beings are rational and will act to advance their own self-interest. See Russell B. Korobkin & Thomas S. Ulen, Law and Behavioral Science: Removing the Rationality Assumption from Law and Economics, 88 Calif. L. Rev. 1051, 1060–61 (2000) (discussing rational choice theory). Yet, psychologists, economists, and other scholars have recognized that, in many circumstances, humans do not behave rationally at all. See, e.g., Oren Bar-Gill, The Behavioral Economics of Consumer Contracts, 92 Minn. L. Rev. 749, 764–65 (2008) (discussing studies revealing bounded rationality through consumer choice); Daniel Kahneman & Amos Tversky, Prospect Theory: An Analysis of Decision Under Risk, 47 Econometrica 263, 268 (1979) (finding that when deciding between two negative options, people prefer a probable—though not certain—loss over a smaller, certain loss). ↑
- Behavioral economists and other social scientists focus on how irrationality undermines our ability to assess accurately the risks we face. See, e.g., Cathy Becker Popescu, Risk and Reason, in Gussow & Thomas, supra note 11, at 374, 376 (observing that often our “risk assessments correlate little with the actual probability of harm” and concluding that “[o]verestimating some risks, while underestimating or discounting others, may engender misplaced fear and misallocation of resources . . . .”). Risk management agendas tend instead to be reactive and consistent with the series of risks that come to the public’s attention. See Stephen Breyer, Breaking the Vicious Circle: Toward Effective Risk Regulation 20 (1993) (“Agency priorities and agendas may more closely reflect public rankings, politics, history . . . than the kind of priority list that environmental experts would deliberately create.”). Citizens and lawmakers alike tend to demand corrective action in the face of “recently materialized” risks of “highly salient” harm. Breyer, supra, at 50 (explaining that congressional and public interest “tends to move the particular problem at issue toward the top of the agency’s agenda”); Christine Jolls et. al., A Behavioral Approach to Law and Economics, 50 Stan. L. Rev. 1471, 1519 (1998). Over time, this trend can lead to over-regulation of “recently materialized” risks of “highly salient” harm, and under-regulation of low-salience harm. Breyer, supra, at 50 (explaining that political pressure from Congress and the public can cause regulators to get “tunnel vision”); Jolls et. al., supra, at 1519. In the context of foodborne illness, “anecdote-driven” regulatory action will likely reflect actual risk poorly. Jolls et. al., supra, at 1518 (noting that inaccurate beliefs about the probability of an event occurring can lead to “anecdote-driven” legislation). ↑
- See Peter B. Hutt & Peter B. Hutt II, A History of Government Regulation of Adulteration and Misbranding of Food, 39 Food. Drug Cosmetic L.J. 2, 53–54 (1984); Part I: The 1906 Food and Drugs Act and Its Enforcement, U.S. Food & Drug Admin., http://www.fda.gov/AboutFDA/WhatWeDo/History/Origin/ucm054819.htm [https://perma.cc/C6MZ-3BQ6]. ↑
- Hutt & Hutt, supra note 95, at 53–54; Part I: The 1906 Food and Drugs Act and Its Enforcement, supra note 95; see Upton Sinclair, The Jungle at Ch. 9 (1906) (describing a food safety system where meat inspectors served the will of the packers and were fired for suggesting practices to preserve safety); id. at Ch. 14 (illustrating vile adulteration with an anecdote describing rat carcasses and bread tainted with rat poison, all found in the meat storage rooms, going into the meat grinders along with meat); see also Pure Food & Drug Act, ch. 3915, §§ 1–5, 34 Stat. 768 (1906) repealed by Federal Food, Drug, and Cosmetic Act of 1938, ch. 675, § 1002(a), 52 Stat. 1059. Note that the Federal Meat Inspection Act was signed into law on the same day as was the Pure Food & Drug Act. See Federal Meat Inspection Act, ch. 2907, 34 Stat. 1260 (1907). ↑
- Peter Barton Hutt, Food Law & Policy: An Essay, 1 J. Food L. & Pol’y 1, 6 (2005). The irony of The Jungle is that Sinclair himself was primarily concerned not with food safety but with child labor and immigrant exploitation in the meatpacking industry. Anthony Arthur, Radical Innocent: Upton Sinclair 83 (2006) (noting that Sinclair famously lamented that he “aimed for the heart, and by accident . . . hit the stomach”). That his work generated legislation in the former area but not the latter reinforces the key point discussed in Part I.B.1 that food safety concerns are compelling. ↑
- Part II: 1938, Food, Drug, Cosmetic Act, U.S. Food & Drug Admin., http://www.fda.gov/AboutFDA/WhatWeDo/History/Origin/ucm054826.htm [https://perma.cc/FQG2-FHXW]. ↑
- Id. ↑
- See Food Additives: Hearings on Bill to Amend the Federal Food, Drug, and Cosmetic Act with Respect to Chemical Additives in Food Before the Subcomm. of the Comm. on Interstate and Foreign Commerce, 85th Cong., 2nd Sess. at 303 (1957); Id. (statement of Jack T. Jennings, Assistant Director, Washington Office, Cooperative League of the U.S.) (arguing that ready-to-eat meals should be “free of dangerous chemicals or other additives”). ↑
- Id. ↑
- See Laws Enforced by FDA, U.S. Food & Drug Admin., https://www.fda.gov/RegulatoryInformation/LawsEnforcedbyFDA/default.htm [https://perma.cc/FBA7-9CRN]. ↑
- Food Bill Aims to Improve Safety, U.S. Food & Drug Admin. (Dec. 23, 2010), https://wayback.archiveit.org/7993/20170405004044/https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm237758.htm [https://perma.cc/7JZV-BLD6]. A 2006 E. coli outbreak traced to spinach was particularly influential on public opinion. See Pollans, Regulating Farming, supra note 4, at 415 (discussing the role of the 2006 outbreak in the history leading up to the FSMA’s passage). ↑
- See Renée Johnson, Cong. Res. Serv., R42885, Food Safety Issues for the 114th Congress 1 (Feb. 13. 2015). ↑
- Id. at 8–9 (describing the FDA’s expanded authority); see Hutt et al., Food and Drug Law: Cases and Materials, supra note 7, at 528 (noting that “the most important pre-FSMA statutory provision for addressing pathogenic contamination of food is [21 U.S.C. § 342(a)(4)], which states that a food is adulterated ‘if it has been prepared, packed, or held under insanitary conditions . . . whereby it may have been rendered injurious to health.’” (quoting § 342(a)(4))); Pollans, Regulating Farming, supra note 4, at 413–17 (describing the history of the FSMA and explaining the shift to prevention of microbial contamination through direct regulation of food handling practices). ↑
- See Pollans, Regulating Farming, supra note 4, at 412–13 (describing FSMA expansion onto farms). ↑
- As food scientist Edwin M. Foster explained when arguing that there is not actually a food safety crisis: “Food is something special. We feel comfortable with food and nervous without it. Concern about our food supply can make us irrational.” Edwin M. Foster, Is There a Food Safety Crisis?, 17 Nutrition Today 6 (1982), reprinted in Gussow & Thomas, supra note 11, at 359. Foster ends his piece with strong language criticizing advocates who have pushed for food safety regulation: “Let’s get our priorities straight. Let’s put our efforts on the real hazards in life and quit dissipating our energies on hypothetical and imaginary dangers . . . .” Id. at 360. ↑
- See infra Table 2 (comparing prevalence of deaths attributable to narrow food safety problems with prevalence of deaths attributable to diet-related disease). ↑
- Cass R. Sunstein, Precautions Against What? The Availability Heuristic and Cross-Cultural Risk Perception, 57 Ala. L Rev. 75, 101 (2005) [hereinafter Sunstein, Precautions Against What?]. For instance, if people can easily call to mind a “vivid and salient” event, “people will have a heightened fear of the risk in question,” even if the risk relates to events that occur infrequently, such as shark attacks. Id. at 77, 93. See also Jolls et. al., supra note 94, at 1519; Daniel Kahneman & Amos Tversky, Judgment Under Uncertainty: Heuristics and Biases, in Judgment and Decision Making: An Interdisciplinary Reader 42–45 (Hal R. Arkes et al., eds., 2d ed., 2000). ↑
- See, e.g., Linda Calvin, U.S. Dept. of Agric., Economic Research Service, Response to U.S. Foodborne Illness Outbreaks Associated with Imported Produce 1–2 (Feb. 2004), https://www.ers.usda.gov/webdocs/publications/42543/19326_aib789-5_1_.pdf?v=0 [https://perma.cc/WMK3-5YPE] (observing that even if the source of an outbreak is an imported item, domestic providers of the same product may suffer from reduced demand). ↑
- See Pollans, Regulating Farming, supra note 4, at 417 (noting that per capita consumption of spinach fell by about 12 percent as a result of the outbreak). For discussion of outbreak effects on consumer trust in tomatoes, see infra note 237–239 and accompanying text. ↑
- Further, once we see that an event has occurred recently, we are prone to attach a high probability to it reoccurring. See Jolls et al., supra note 94, at 1519. ↑
- Sunstein, Precautions Against What?, supra note 109, at 77. ↑
- See, e.g., Eric Jéquier, Pathways to Obesity, 26 Int’l. J. of Obesity S12, S15 (2002) (finding that environmental and behavioral changes contribute to obesity); see also infra note 141 (discussing how challenges of causation make it difficult to place blame on individual marketers of unhealthy foods). ↑
- See Kahneman & Tversky, supra note 93, at 268–69 (arguing that people overvalue the certainty of an outcome and undervalue the probability of an outcome). ↑
- See Adam Benforado et al., Broken Scales: Obesity and Justice in America, 53 Emory L. J. 1645, 1684–86 (2004) (discussing the relationship between our modern dietary preferences and evolution). ↑
- Id. at 1685–86 (discussing our preferences for fats, sugars, and salts); see, e.g., Natascha Loebnitz & Klaus G. Grunert, Impact of Abnormally Shaped Vegetables on Consumers’ Risk Perception, 63 Food Quality & Preference 80, 84 (2018) (finding that people “expressed higher risk perceptions for abnormally-shaped vegetables” than for “normally-shaped ones”). ↑
- See Charlotte Fabiansson & Stefan Fabiansson, Food And The Risk Society: The Power of Risk Perception 11 (2016) (noting that “familiarity with food products may influence how information about the risks . . . of some food is conveyed”). Cognitive psychologists call this the “mere exposure effect”: humans may develop positive feelings towards things to which they are repeatedly exposed. Robert F. Bornstein, Exposure and Affect: Overview and Meta-Analysis of Research, 1968–1987, 106 Psychol. Bull. 265, 265 (1989). Once we develop these pleasurable associations, we are not likely to fear that thing. See Paul Slovic et. al., The Affect Heuristic, 177 Eur. J. of Operational Res. 1333, 1335 (2007). ↑
- See Deon Klerck & Jillian C. Sweeney, The Effect of Knowledge Types on Consumer-Perceived Risk and Adoption of Genetically Modified Foods, 24 Psychol. & Marketing 171, 171 (2007) (discussing the relationship between consumer risk perception and consumer demand in the context of genetically modified foods); Loebnitz & Grunert, supra note 117. ↑
- See Gary P. Nabhan, Why Some Like It Hot: Food, Genes, and Cultural Diversity 175–76 (2004). ↑
- Arye Lev-Ran, Human Obesity: An Evolutionary Approach to Understanding our Bulging Waistline, 17 Diabetes Metabolism Res. Rev. 347, 353–54 (2001) (explaining that obesity has “only quite recently . . . stopped being a sign of wealth” and started being “a social liability”). ↑
- Nabhan, supra note 120, at 177. The evolutionary aspect of this problem is not within the realm of behavioral economics; we nevertheless discuss it here because it is a feature of human evolutionary development that may contribute to current non-rational regulatory decision-making. ↑
- See Ted O’Donoghue & Matthew Rabin, Doing It Now or Later, 89 Am. Econ. Rev. 103, 118 (1999) (finding that less sophisticated people tend to consume tempting goods immediately if the costs of this consumption decision are delayed). One study asked subjects to choose between receiving a smaller reward earlier and a larger reward later. Kris N. Kirby & R.J. Herrnstein, Preference Reversals Due to Myopic Discounting of Delayed Reward, 6 Psychol. Sci. 83, 84–85 (1995). Although all subjects indicated that they preferred the larger, later reward when the delays to both rewards were long, subjects consistently reversed their choice, and picked a smaller, earlier reward when delays to both rewards were shortened. Id. at 85–87. ↑
- See Jon D. Hanson & Douglas A. Kysar, Taking Behavioralism Seriously: The Problem of Market Manipulation, 74 N.Y.U. L. Rev. 630, 679 (1999) (“Today we believe that we should stop smoking or diet tomorrow, but tomorrow we feel we should continue smoking or overeating, at least for another day.”). ↑
- A 2017 study by the firm Market Research estimated the value of the diet industry at $66 billion. U.S. Weight Loss Market Worth $66 Billion, PR Newswire (Dec. 20, 2017), https://www.prnewswire.com/news-releases/us-weight-loss-market-worth-66-billion-300573968.html [https://perma.cc/TZ65-3H9R]. ↑
- Cass R. Sunstein, Terrorism and Probability Neglect, 26 J. Risk & Uncertainty 121, 121–22 (2003) (describing the relationship between irrational fear and perceived level of control). The perception that individuals have control over diet choices also contributes to paternalism-based objections to nutrition regulation. See infra note 128 and accompanying text. ↑
- See Biber, supra note 8, at 12 (explaining that when an agency has multiple goals, it will likely prioritize “easily measured goals” over others). ↑
- See Lawrence O. Gostin, Bloomberg’s Health Legacy: Urban Innovator or Meddling Nanny?, 43 Hastings Ctr. Rep. 19, 20–21 (2013) (describing “nanny state” critiques of New York City nutrition policies, addressing menu labeling, sodium reduction, soda portion controls, and a trans-fat ban); Lindsay F. Wiley et al., Who’s Your Nanny? Choice, Paternalism and Public Health in the Age of Personal Responsibility, 41 J.L. Med. & Ethics 88, 88–89 (2013) (exploring “public health paternalism”). ↑
- U.S. Food & Drug Admin., Analysis of Economic Impacts – Standards for the Growing, Harvesting, Packing and Holding of Produce for Human Consumption 29–31 (2013), http://www.fda.gov/downloads/Food/FoodSafety/FSMAUCM334116.pdf [https://perma.cc/A6AW-YJ7R] [hereinafter Economic Analysis of Produce Safety Rule] (estimating how many farmers were already subject to private governance in order to determine how much compliance with the FSMA would cost). ↑
- The FDA and USDA established the GAP program in 1998. Id. at 35. See also U.S. Food & Drug Admin., Guidance For Industry: Guide To Minimize Microbial Food Safety Hazards For Fresh Fruits And Vegetables 1 (1998), https://www.fda.gov/downloads/Food/GuidanceRegulation/UCM169112.pdf [https://perma.cc/B8K3-DQ4Y] (establishing voluntary science-based guidance in response to a request from the President to develop best practices for produce); Good Agricultural Practices (GAP) & Good Handling Practices (GHP), U.S. Dep’t of Agric., https://www.ams.usda.gov/services/auditing/gap-ghp [https://perma.cc/R7L8-EYEP] (describing the USDA GAP program as a system of voluntary audits that conform to the FDA and USDA Guidance for Industry). ↑
- Economic Analysis of Produce Safety Rule, supra note 129, at 35–36 (noting a variety of reasons why a farm might be GAP compliant but not USDA GAP certified). ↑
- Pollans, Regulating Farming, supra note 4, at 414 & 415 n.65 (noting that Safeway was “the first retailer to impose this requirement,” and that “Albertson’s followed suit shortly thereafter” (citing Kenneth S. Petersen, Third-Party Audit Programs for the Fresh-Produce Industry, in Microbial Safety of Fresh Produce 322 (Xuetong Fan et al. eds., 2009)). ↑
- Economic Analysis of Produce Safety Rule, supra note 129, at 36. Tomato farmers in Florida are subject to a state produce safety regulation requiring compliance with the Tomato Best Practices Manual. Id. at 31–32. ↑
- Id. at 37. ↑
- Id. at 38. ↑
- Id. at 33–34. A leafy green marketing agreement is a quasi-public form of regulation that imposes obligations on participating handlers, requiring them to purchase leafy greens only from producers that have passed LGMA audits. Pollans, Regulating Farming, supra note 4, at 415 n.67 (explaining that although marketing agreements function like private contracts, the USDA plays a facilitating role pursuant to the Agricultural Marketing Agreement Act of 1937). ↑
- See Tacy Katherine Hass, New Governance: Can User-Promulgated Certification Schemes Provide Safer, Higher Quality Food?, 68 FOOD & DRUG L.J. 77, 86 (2013) (discussing the National Restaurant Association and Grocery Manufacturer’s Association’s support for the FSMA, and identifying their interest in uniformity as one such reason). ↑
- See Pollans, Regulating Farming, supra note 4, at 416–17 (noting that the leafy greens industry supported a national leafy green marketing agreement before it supported the Food Safety Modernization Act). ↑
- There are a number of private governance schemes addressing both the workplace and the environment, but, unlike traditional food safety private governance, they are more piecemeal, operate either via voluntary certifications or through individual businesses, and tend to rely on consumer willingness to opt in and pay more for a certified product. See Stephen Lee, The Food We Eat and the People Who Feed Us, 94 Wash. U.L. Rev. 1249, 1273 (2017) (discussing the limitations of private governance in the context of fair wages and workplace safety); Czarnezki, Pollans & Main, supra note 86, at 1008–21 (discussing the limitations of eco-labeling certification schemes). ↑
- Strict liability applies to the sale or distribution of defective food products. See, e.g., Jackson v. Nestle-Beich, Inc., 589 N.E.2d 547, 550 (Ill. 1992) (holding that “strict liability . . . is intended to apply to all products placed in the stream of commerce regardless whether they have undergone some processing or not”); Restatement (Second) of Torts § 402A cmt. d (1965) (“The rule stated in this Section is not limited to the sale of food for human consumption, or other products for intimate bodily use, although it will obviously include them.”). ↑
- See, e.g., Pelman v. McDonald’s Corp., 237 F. Supp. 2d 512, 520, 538–40 (S.D.N.Y. 2003) (dismissing plaintiffs’ claim that McDonald’s acted negligently “in selling food products that are high in cholesterol, fat, salt and sugar when studies show that such foods cause obesity and detrimental health effects” in part on the grounds that the complaint failed to plead sufficient facts to establish causation); see also Pollans, Drinking Water Protection, supra note 65, at 1238–48 (describing limits of existing law to hold agricultural polluters accountable for drinking water contamination). ↑
- See Alexia Brunet Marks, Check Please: Using Legal Liability to Inform Food Safety Regulation, 50 Hous. L. Rev. 723, 724 (2013) (discussing how foodborne illness litigation incentivizes firms “to increase food safety practices”). There is a growing market for food safety liability insurance, and some retail and institutional buyers have begun requiring producers, particularly those exempt from the FSMA, to carry policies. See John Aloysius Cogan Jr., The Uneasy Case for Food Safety Liability Insurance, 81 Brook. L. Rev. 1495, 1498–99 (2016). ↑
- K.L. Newman et al., The Impact of Socioeconomic Status on Foodborne Illness in High-Income Countries: A Systematic Review, 143 Epidemiol. Infect. 2473, 2479 (2015) (finding some correlation between specific foodborne pathogens and socioeconomic status); see also Jennifer J. Quinlan, Foodborne Illness Incidence Rates and Food Safety Risks for Populations of Low Socioeconomic Status and Minority Race/Ethnicity: A Review of the Literature, 10 Int. J. Envtl. Res. Pub. Health 3634, 3637 (2013) (finding some correlation between incidence of foodborne illness and racial and ethnic status). ↑
- See Newman et al., supra note 143, at 2475–76, 2479 (finding that “high SES was associated with increased incidence of campylobacteriosis and salmonellosis”); see also Bridget M. Whitney et. al., Socioeconomic Status and Foodborne Pathogens in Connecticut, USA, 2000–2011, 21 Emerging Infectious Diseases 1617, 1619 (2015) (noting higher rates of Salmonella illness among Connecticut residents with higher socioeconomic status). The “likely explanation” for why foodborne illnesses like Salmonella are more prevalent among wealthier individuals is that they are more likely than lower-income individuals to travel internationally and “eat[ ] at restaurants”—both activities of which present major risk factors for Salmonella. Id. at 1621; see also Newman et al., supra note 143, at 2479 (“Risk factors for Campylobacter include eating restaurant-prepared food, having contact with farm animals, drinking untreated surface water, eating undercooked food, and drinking raw milk. Many of these risk factors are associated with higher [socioeconomic status] groups.” (internal citations omitted)). ↑
- Newman et al., supra note 143, at 2479 (finding that “[l]ow SES was associated with increased incidence of listeriosis”). “Risk factors for Listeria include eating cold processed meats, unpasteurized milk products, and being immunosuppressed.” Id. (citing Bala Swaminathan & Peter Gerner-Smidt, The Epidemiology of Human Listeriosis, 9 Microbes & Infection 1236 (2007)). These particular risk factors of listeriosis (with the exception of consuming raw milk) are associated with individuals from lower socioeconomic classes. Id. at 2479–80. ↑
- A. Espelt et al., Socioeconomic Inequalities in Diabetes Mellitus Across Europe at the Beginning of the 21st Century, 51 Diabetologia 1971, 1974–75 (2008) (finding that low socioeconomic position was correlated with higher rates of diabetes and higher diabetes mortality rates); Doreen M. Rabi et. al., Association of Socio-Economic Status with Diabetes Prevalence and Utilization of Diabetes Care Services, 6 BMC Health Serv. Research 124, 124 (2006) (“Significant socio-economic gradients have been shown in the prevalence of several cardiovascular disease risk factors, including diabetes.”). ↑
- See Pollans, Regulating Farming, supra note 4, at 405–06 & 406 n.24 (citing sources describing the environmental justice problems associated with CAFOs). ↑
- See, e.g., 25 Leading Global Companies Join to Accelerate Transformational Change in Global Food Systems, World Bus. Council for Sustainable Dev., (Jan. 19, 2017), https://www.wbcsd.org/Programs/Food-Land-Water/Food-Land-Use/FReSH/News/25-leading-global-companies-join-together-to-accelerate-transformational-change-in-global-food-systems [https://perma.cc/FD4Y-F2KR] (describing a collaborative project between members of the World Business Council for Sustainable Development and members of the EAT Foundation to improve diet-related health outcomes). ↑
- Perhaps one reason for this disconnect is that nutrition is often framed as a matter of personal choice rather than as a market failure. Thus, regulatory intervention is characterized as unnecessarily paternalistic. Wiley, supra note 128, at 88–89 (exploring arguments that nutrition regulation is paternalistic). ↑
- Estimates of Foodborne Illness in the United States, Ctrs. for Disease Control & Prevention, http://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html [https://perma.cc/78NV-JA42.]. ↑
- Tobenna D. Anekwe & Sandra Hoffmann, U.S. Dept. of Agric., Economic Research Serv., Making Sense of Recent Cost-of-Foodborne-Illness Estimates 1 (Sept. 2013), https://www.ers.usda.gov/webdocs/publications/43796/40344_eib118.pdf?v=0 [https://perma.cc/8C3J-6VS7]. The broad range in estimates follows in part from the fact that the estimates do not consider the same set of illnesses. Id. at 12. ↑
- Xu et al., supra note 42, at 6 tbl.B. ↑
- Id. ↑
- Ctrs. for Disease Control & Prevention, National Diabetes Statistics Report, 2017: Estimates of Diabetes and Its Burden in the United States 2, 7 (2017), https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf [https://perma.cc/95EN-Q5PG] (clarifying that the statistic includes both Type 1 and Type 2 diabetes but that Type 2 accounts for 90% to 95% of all cases). ↑
- CDC, Adult Obesity Causes, supra note 42. ↑
- Overweight and Obesity Statistics, Nat’l Inst. of Health, Nat’l Inst. of Diabetes and Digestive and Kidney Disease, https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity [https://perma.cc/59HW-6XDL]. ↑
- The Cost of Diabetes, Am. Diabetes Ass’n, http://www.diabetes.org/advocacy/news-events/cost-of-diabetes.html [https://perma.cc/J7VQ-L4SB] (research considers both Type 1 and Type 2 diabetes). ↑
- Emilia J. Benjamin et al. Heart Disease and Stroke Statistics—2018 Update: A Report from the American Heart Association, 137 Circulation e67, e40 (2018) (offering average annual costs from 2013–2014); see also Alan S. Go et al., American Heart Association, Heart Disease & Stroke Statistics—2014 Update: A Report from the American Heart Association at e280 (2014), http://circ.ahajournals.org/content/129/3/e28 [http://perma.cc/QY99-MB42]. ↑
- Go, supra note 158, at e147. ↑
- . Robert L. Scharff, Economic Burden from Health Losses Due to Foodborne Illness in the United States, 75 J. Food Prot. 123, 123 (2012). ↑
- Id. ↑
- Id. at 128. The table shows a midrange cost estimate. Other studies offer estimate ranges from $14.2 to $152 billion. See supra note 151 and accompanying text; see also Sandra Hoffman, Bryan Maculloch & Michael Batz, U.S. Dep’t of Agric., Econ. Research Serv., Economic Burden of Major Foodborne Illnesses Acquired in the United States 14 (2015) https://www.ers.usda.gov/webdocs/publications/43984/52807_eib140.pdf?v=0 [https://perma.cc/L4QJ-PWTY] (putting the total cost at $15 billion, as the mean annual economic costs related to the fifteen major pathogens that cause 95 percent of the disease burden in the United States). ↑
- Ctrs. for Disease Control & Prevention, National Diabetes Statistics Report, supra note 154, at 10. This number includes only instances where diabetes was listed as the underlying cause of death on death certificates; however, it is one of several underlying or contributing causes of death in over 252,000 additional cases. Id. ↑
- Id. at 1. This is the estimated number of people living with diabetes in 2015; it is not the number of new cases. Id. ↑
- Wenya Yang et al., American Diabetes Association, Economic Costs of Diabetes in the U.S. in 2017, 41 Diabetes Care 917, 924 (2018), http://care.diabetesjournals.org/content/diacare/early/2018/03/20/dci18-0007.full-text.pdf [https://perma.cc/PJ4D-URPW]. ↑
- Heart Disease Fact Sheet, Ctrs. For Disease Control & Prevention, https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_heart_disease.htm [https://perma.cc/N2J3-PY2G] (last updated Aug. 23, 2017) [hereinafter Heart Disease Fact Sheet]. ↑
- nat’l Ctr. for health Statistics, Summary Health Statistics: National Health Interview Survey, 2016 at 4 tbl.A-1b (measuring the frequency of heart disease among adults aged eighteen and over in the United States), https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_A-1.pdf [https://perma.cc/PL3Y-F8PL]. ↑
- Heart Disease Fact Sheet, supra note 166. ↑
- We assume, for the purposes of this analysis, that it is normatively desirable for the scale of public intervention to be proportionate to the scale of a public health problem. ↑
- See U.S. Gov’t Accountability Office, GAO-18-174, Food Safety And Nutrition: FDA Can Build On Existing Efforts To Measure Progress And Implement Key Activities 22 (2018), https://www.gao.gov/assets/690/689796.pdf [https://perma.cc/VGD6-XNMQ]; U.S. Food & Drug Admin., Justification of Estimates For Appropriations Committees 12 (2017), https://www.fda.gov/media/106463/download [ https://perma.cc/C6SV-PLN8]. ↑
- See U.S. Gov’t Accountability Office, GAO-18-174, supra note 170, at 22. The amount spent on nutrition may, however, be even lower, as the FDA included expenditures on implementing food allergen labeling, arguably an issue of food safety, in its nutrition-related staffing and costs. See id. at 27. ↑
- See id. at 22. ↑
- Id. at 15, 42 (defining “key” as “most relevant to and substantive for this review”). ↑
- Id. at 15. ↑
- Id. at 28. ↑
- See id. at 31; see also id. at 29 tbl.3 (outlining the goal, outcome, and objectives for food safety-related activities and nutrition-related activities). FDA’s nutrition-related priorities include issuing guidance to help implement menu labeling regulations and the updated Nutrition Facts label requirements, as well as implementing new requirements for use of the term “healthy” on food labels. U.S. Food & Drug Admin., Healthy Innovation, Safer Families: FDA’s 2018 Strategic Policy Roadmap 15 (Jan. 2018), https://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Reports/UCM592001.pdf [https://perma.cc/AE8S-P8YT]. These action items appear under the header “Leveraging Diet and Nutrition to Reduce Preventable Death and Disease.” Id. at 7. This was one of the subcategories of the priority area described, “Empower consumers to make better and more informed decisions about their diets and health; and expand the opportunities to use nutrition to reduce morbidity and mortality from disease.” Id. ↑
- U.S. Gov’t Accountability Office, GAO-18-174, supra note 170, at 52–53. ↑
- Id. at 32. ↑
- See generally id. Here, the FDA did not specifically enumerate its accomplishments related to nutrition; however, it seems that such activities fell into a category entitled “Promote Informed Decisions,” which included, along with non-nutrition accomplishments, finalizing guidance on menu labeling of caloric information, issuing a request for information on use of the term “healthy” on food products, and issuing draft guidance on voluntary sodium reduction. Id. at 48–52. ↑
- U.S. Dep’t of Agric., FY 2018 Budget Summary 48 tbl. FdS-1 (2017), https://www.usda.gov/sites/default/files/documents/USDA-Budget-Summary-2018.pdf [https://perma.cc/59EL-XF7B] [hereinafter USDA FY 2018 Budget Summary] (showing total 2016 budget expenditures of $1.27 billion for the FSIS, the agency within the USDA tasked with overseeing food safety); Johnson, supra note 1, at 1 (describing the scope of the FSIS’s authority). ↑
- See id. at 40 tbl. FNCS-2 (categorizing USDA Food and Nutrition Service (FNS) spending for 2016). ↑
- See id. at 39. The FNS includes the Center for Nutrition Policy and Promotion and the Food and Nutrition Service. Id. ↑
- See Supplemental Nutrition Assistance Program (SNAP), U.S. Dep’t of Agric. Food and Nutrition Serv., https://www.fns.usda.gov/snap/eligibility [https://perma.cc/3CUK-RBH8] (explaining SNAP program benefits). ↑
- USDA Fy 2018 Budget Summary, supra note 180, at 40 tbl. FNCS-2. ↑
- What Can SNAP Buy?, U.S. Dep’t of Agric. Food and Nutrition Serv., https://www.fns.usda.gov/snap/eligible-food-items [https://perma.cc/3MYE-XWXX]. In 2018, the USDA rejected an application from the State of Maine seeking to exclude soda from the SNAP program. The USDA explained that it “[did not] want to be in the business of . . . passing judgment about the relative benefits of individual food products.” Caitlin Dewey, Why the Trump Administration Won’t Let Maine Ban Soda and Candy from Food Stamps, Wonkblog, Wash. Post (Jan. 20, 2018), https://www.washingtonpost.com/news/wonk/wp/2018/01/20/why-the-trump-administration-wont-let-maine-ban-soda-and-candy-from-food-stamps/?utm_term=.0bf49b13a83d [https://perma.cc/L4U9-NG9J] (quoting a statement from the USDA). ↑
- One example of this phenomenon is the Special Supplemental Nutrition Program for Women, Infants, and Children, which provides benefits to purchase specific foods that the USDA has determined are essential for these demographic categories. See WIC Food Packages - Maximum Monthly Allowances, U.S. Dep’t of Agric. Food and Nutrition Serv., https://www.fns.usda.gov/wic/wic-food-packages-maximum-monthly-allowances [https://perma.cc/R8ZH-X9ZR] (showing a chart for specific food products and amounts for each category of WIC participant). ↑
- U.S. Dep’t Of Agric., Supplemental Nutrition Assistance Program Education (SNAP-Ed) Budget Allocation For Fiscal Year 2017 (2017), https://snaped.fns.usda.gov/snap/Guidance/SNAP-EdBudgetAllocationFY2017.pdf [https://perma.cc/KXK3-JKU9]. ↑
- See About SNAP-Ed Connection, U.S. Dep’t of Agric., https://snaped.fns.usda.gov/about#snaped [http://perma.cc/HAJ4-B888]. ↑
- Press Release, Release No. 0143.16, U.S. Dep’t of Agric., Food & Nutrition Serv., Agriculture Secretary Vilsack Announces $16.8 Million in Grants to Encourage Healthy Food Purchases for SNAP Participants (June 8, 2016), https://www.fns.usda.gov/pressrelease/2016/014316 [https://perma.cc/K2MW-GEG6]. ↑
- See Healthy Food Financing Initiative, U.S. Dep’t Of Health & Human Services, Off. Of Community Services, https://www.acf.hhs.gov/ocs/programs/Community-Economic-Development/Healthy-Food-Financing [https://perma.cc/H9Y7-EKSN]. ↑
- This is, of course, a very rough estimate in part because our statistics on lives lost do not include data on the intermediate component of traditional food safety: carcinogenic food additives. We exclude these numbers in part because they could arguably be classified as either traditional food safety (when cancer is caused by repeated ingestion of a particular food additive) or nutrition (when cancer is caused by general overconsumption of processed foods). One key exception to this mismatch is in federally sponsored research. At the NIH and the CDC, spending tracks actual risk, at least as between traditional food safety and nutrition. In 2016, NIH invested over $1 billion in diabetes research and $1.2 billion in heart disease research. Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC), NIH (May 18, 2018), https://report.nih.gov/categorical_spending.aspx [https://perma.cc/9SUT-MX24]. By contrast, NIH spent only $116 million on foodborne illness research. Id. Similarly, in 2016, the CDC spent over $170 million on diabetes but only $21.5 million on foodborne illness. Ctrs. for Disease Control & Prevention, Justification of Estimates for Appropriation Committees, Fiscal Year 2018 93 (2017), https://www.cdc.gov/budget/documents/fy2018/fy-2018-cdc-congressional-justification.pdf [https://perma.cc/8D9V-YYVK]. ↑
- Cigarettes, U.S. Food & Drug Admin., https://www.fda.gov/TobaccoProducts/Labeling/ProductsIngredientsComponents/ucm482563.htm [https://perma.cc/F5CZ-QLUD] (“Cigarettes are responsible for the vast majority of all tobacco-related disease and death in the United States . . . . The harmful chemicals in cigarette smoke can damage nearly every organ in the body.”) ↑
- See, e.g., Am. Beverage Ass’n v. City & Cty. of San Francisco, No. 16-16073 (9th Cir. 2019) (en banc) (Ikuta, J., concurring in part and dissenting in part) (arguing that a mandatory warning label on advertisements of sugar-sweetened beverages could not withstand a first amendment challenge because it is not “factual and uncontroversial” that sugar-sweetened beverages pose unique health risks for purposes of the Zauderer test); see also Josef Weimholt, “Bringing A Butter Knife to A Gun Fight”? Salience, Disclosure, and FDA’s Differing Approaches to the Tobacco Use and Obesity Epidemics, 70 Food & Drug L.J. 501, 521–22 (2015) (contrasting FDA approaches to tobacco and obesity, noting that in both contexts the primary tool is information disclosure, but the FDA approach to tobacco warnings is far more visceral and directly aimed at encouraging behavioral change rather). ↑
- See, e.g., Deborah L. Rhode, Obesity and Public Policy: A Roadmap for Reform, 22 Va. J. Soc. Pol’y & L. 491, 501–03, 507 (2015) (describing the primary opposition to obesity policies such as sugar-sweetened beverage taxes as because they are paternalistic or evidence a “nanny state,” and noting that intensive lobbying efforts resulted in repeal of excise taxes on soft drinks and defeat of new attempted measures). ↑
- See 21 U.S.C. §§ 341–350l-1 (2012). For a more detailed description of traditional food safety regulation, see supra Part I.A.1 & 2. ↑
- See Weimholt, supra note 193, at 503 (arguing that the FDA’s primary approach to obesity has been through information-based regulatory tools); see also FDA, Draft Sodium Guidance, supra note 52. ↑
- See Cass R. Sunstein, Health-Health Tradeoffs, 63 U. Chi. L. Rev. 1533, 1535 (1996) [hereinafter Sunstein, Health-Health Tradeoffs] (describing health-health tradeoffs as “a pervasive problem in risk regulation”); see also Rascoff & Revesz, supra note 5, at 1763–65 (discussing tradeoffs in government decision-making). ↑
- Sunstein, Health-Health Tradeoffs, supra note 197, at 1535 (defining “health-health tradeoffs” as “when the diminution of one health risk simultaneously increases another health risk”). “[F]or example, fuel economy standards, designed to reduce environmental risks, may make automobiles less safe, and in that way increase risks to life and health.” Id. ↑
- U.S. Dep’t of Agric., Food Safety Information: Clostridium Botulinum 2 (Jan. 2010) (explaining how nitrates and nitrites are used to prevent botulism in meat and poultry products), https://www.fsis.usda.gov/wps/wcm/connect/a70a5447-9490-4855-af0d-e617ea6b5e46/Clostridium_botulinum.pdf?MOD=AJPERES [https://perma.cc/7XTB-MZPN]. ↑
- Id. at 3 (explaining that this risk can be mitigated with proper food processing practices). ↑
- See Sunstein, Health-Health Tradeoffs, supra note 197, at 1540 (noting that “[a] well-functioning administrative state [will] seek a measure of coordination among agencies”). ↑
- See generally Pollans, Regulating Farming, supra note 4, at Part I.B. (discussing tradeoffs in produce safety regulation). Critics also suggest that FDA overvalues the benefits of strengthening narrow food safety regulation. See Andy Weisbecker, More or Less Food Safety Regulation?, Food Safety News (Mar. 11, 2010) (explaining that opponents of the FSMA believe that it “favors an industrial agricultural system, and . . . local food systems provide significant food safety benefits”), http://www.foodsafetynews.com/2010/03/more-or-less-food-safety-regulation/#.WoWTM6inHD4 [https://perma.cc/2URV-E72L]. ↑
- In addition, in the FSMA context, discussion of the unintended consequences is, by necessity, speculative. FDA regulations offer farmers and food processors many choices; it is still too early to tell how regulated businesses will respond. The regulations were finalized recently, and many of the compliance deadlines are still several years off. Accordingly, it will be many years before it will be possible empirically assess the statute’s actual public health costs and benefits. ↑
- See FDA Food Safety and Modernization Act (FSMA) § 105, 21 U.S.C. § 350h (2012). ↑
- See U.S. Food & Drug Admin., Part 117: FSMA Final Rulemaking for Current Good Manufacturing Practice and Hazard Analysis and Risk-Based Preventive Controls for Human Food 40–41 (2015) (noting that economic analysis of the proposed rule’s potential health benefits will consider morbidity and mortality effects of foodborne illnesses and lost health); U.S. Food & Drug Admin., FDA-2011-N-0921, Standards for the Growing, Harvesting, Packing and Holding of Produce for Human Consumption, Final Regulatory Impact Analysis 4 (2015) [hereinafter Produce Safety Final RIA] (acknowledging that implementation of the rule “will lead to higher costs for both the industry and consumers than the current state of no new regulatory action”). ↑
- See U.S. Dept. of Health & Human Servs. & U.S. Dept. of Agric., Dietary Guidelines for Americans, 2015-2020 xiii (8th ed. 2015), https://health.gov/dietaryguidelines/2015/resources/2015-2020_Dietary_Guidelines.pdf [https://perma.cc/U2J2-9GDZ] (suggesting increased consumption of fruits, vegetables, and whole grains and decreased consumption of saturated fats, sugars, and trans fats). ↑
- Robert H. Lustig et al., The Toxic Truth About Sugar, 482 Nature 27, 28 (2012). ↑
- For fruit, the calories available daily rose from 81.9 to 94 between 1970 and 2015. U.S. Dep’t of Agric., Loss Adjusted Calories Per Capita: Fruit (2017), https://www.ers.usda.gov/webdocs/DataFiles/50472/Fruit.xls?v=42942 [https://perma.cc/B4ZU-P3JZ]. For vegetables, the calories available has held relatively steady from 129.3 to 130.7 between 1970 and 2015. U.S. Dep’t of Agric., Loss Adjusted Food Availability: Vegetables (2017), https://www.ers.usda.gov/webdocs/DataFiles/50472/veg.xls?v=42942 [https://perma.cc/G9P2-XM4G]. ↑
- See Pablo Monsivais et al., Following Federal Guidelines to Increase Nutrient Consumption May Lead to Higher Food Costs for Consumers, 30(8) Health Aff. 1471, 1471 (2011) (noting that “[n]utrient-dense foods tend to cost more than [calorie-dense foods with] minimal nutritional value”); Mayuree Rao et al., Do Healthier Foods and Diet Patterns Cost More Than Less Healthy Options? A Systematic Review & Meta-analysis, 3 BMJ Open 1, 11 (2013) (conducting a metanalysis of studies assessing price differentials and finding that “[o]n average, healthier food-based diet patterns were more expensive than less healthy patterns”). ↑
- David Wallinga, Agricultural Policy and Childhood Obesity: A Food Systems and Public Health Commentary, 29(3) Health Aff. 405, 407 (2010). ↑
- See Tatiana Andreyeva et al., The Impact of Food Prices on Consumption: A Systematic Review of Research on the Price Elasticity of Demand for Food, 100(2) Am. J. of Pub. Health 216, 218 (2010) (finding that while overall food price elasticity is low, fruit has higher price elasticity than other categories such as eggs, cheese, and sweets); Catherine Durham & James Eales, Demand Elasticities for Fresh Fruit at the Retail Level, 42 Applied Econ. 1345, 1350 (2010) (finding higher price elasticity, or change in demand in response to a change in price, for fruit than had previously been found). ↑
- Emily Broad Leib, The Forgotten Half of Food System Reform: Using Food and Agricultural Law to Foster Healthy Food Production, 9 J. Food L. & Pol’y 17, 35 (2013); see supra notes 62–64 and accompanying text (discussing the Dietary Guidelines). ↑
- Produce Safety Final RIA, supra note 205, at 117. In its preliminary economic analysis, the FDA went so far as to suggest that farmers make behavioral changes to offset the costs of complying with the Produce Safety Rule, such as increasing their off-farm income in order to better support their farms. See Economic Analysis of Produce Safety Rule, supra note 129, at 318 (“FDA believes farm operators are likely to make behavioral adjustments that would alleviate the impact of a regulation on their net returns. Farm operators may decide to increase their off-farm income . . . Farms may also learn to comply with the regulation more cost-effectively over time.”). ↑
- Produce Safety Final RIA, supra note 205, at 7. (arguing that the “exemptions are set up in such a way as to encourage sales of produce locally” but not offering any analysis or justification for the assertion that local produce sales will actually increase as a result of the rule). ↑
- Id. at 11. ↑
- Id. ↑
- The Produce Safety Rule Environmental Impact Statement mentions and immediately rejects this concern on the ground that FDA expects other growers to step in to meet demand. U.S. Food & Drug Admin., FDA-2014-N-2244, Final Environmental Impact Statement for the Proposed Rule: Standards for Growing, Harvesting, Packing, and Holding of Produce for Human Consumption, at 5-23 (Oct. 2015) [hereinafter Produce Safety Rule Final EIS] (offering no justification for this conclusion). ↑
- Produce Safety Rule Final RIA, supra note 205, at 29–30 tbl. 2 (estimating total costs at $560.19 million annually); see also Produce Safety Rule Final EIS, supra note 217, at 4-8, 5-27 (considering as possible human health costs of the proposed rule only costs associated with chemical treatment agricultural waters). ↑
- See, e.g., Patrick Baur et al., Inconsistent Food Safety Pressures Complicate Environmental Conservation for California Produce Growers, 70 Cali. Agric. 142 (2016); Daniel S. Karp et al., The Unintended Ecological and Social Impacts of Food Safety, 65 BioScience 1173, 1178–80 (2015) (surveying various environmental consequences of standard food safety practices); Pollans, Regulating Farming, supra note 4, at 420–27 (describing conflicts between food safety law and environmental best practices). ↑
- ReFED, A Roadmap to Reduce U.S. Food Waste by 20 Percent 16 (2016), https://www.refed.com/downloads/ReFED_Report_2016.pdf [https://perma.cc/557D-LC2G] (estimating that 62.5 million tons (125 billion pounds) of food are wasted per year in the US). ↑
- Gunders, supra note 74, at 10. ↑
- ReFED, A Roadmap to Reduce U.S. Food Waste by 20 Percent: Technical Appendix 65 (2016), https://www.refed.com/downloads/ReFED_Technical_Appendix.pdf [https://perma.cc/23UG-KWXS] (estimating that wasted food utilizes 21 percent of freshwater, 19 percent of fertilizer, 18 percent of cropland, and 21 percent of landfill). ↑
- Gunders, supra note 74, at 30. ↑
- ReFED, supra note 220, at 50. ↑
- Kumar Venkat, The Climate Change and Economic Impacts of Food Waste in the United States, 2(4) Int’l J. on Food System Dynamics 431, 444 (2011). ↑
- See, e.g., Food Recovery Challenge (FRC), U.S. Envtl Protection Agency, https://www.epa.gov/sustainable-management-food/food-recovery-challenge-frc [https://perma.cc/8NSJ-8KKQ] (describing EPA’s Food Recovery Challenge); Selected New and Ongoing USDA Food Loss and Waste Reduction Activities, U.S. Dep’t of Agric., https://www.usda.gov/foodwaste/activities [https://perma.cc/NH7E-ML49] (describing more than a dozen USDA programs and initiatives aimed at reducing food waste); United States Food Loss and Waste 2030 Champions, U.S. Envtl Protection Agency, https://www.epa.gov/sustainable-management-food/united-states-food-loss-and-waste-2030-champions [https://perma.cc/BX3G-Y5CM] (explaining the joint USDA and EPA 2030 Food Waste Champions program); Press Release, U.S. Dep’t of Agric., USDA and EPA Launch U.S. Food Waste Challenge (June 4, 2013), https://www.usda.gov/media/press-releases/2013/06/04/usda-and-epa-launch-us-food-waste-challenge [https://perma.cc/47PU-NBTD] (describing the launch of the USDA and EPA US Food Waste Challenge). ↑
- Press Release, U.S. Dep’t of Agric., USDA and EPA Join Private Sector, Charitable Organizations to Set Nation’s First Food Waste Reduction Goals (Sept. 16, 2015), https://www.usda.gov/wps/portal/usda/usdamediafb?contentid=2015/09/0257.xml&printable=true [https://perma.cc/MU75-KKCM]. ↑
- Emily M. Broad Leib et. al, Harvard Law School Food Law & Policy Clinic, Food Safety Regulations & Guidance for Food Donations: A 50-State Survey of State Practices 1 (2018), https://www.chlpi.org/wp-content/uploads/2013/12/50-State-Food-Regs_March-2018_V2.pdf [https://perma.cc/5RP4-BNRN] (“A key barrier to the donation of surplus food is the lack of knowledge or readily available guidance regarding safety procedures for food donation.”). ↑
- News Release, U.S. Food & Drug Admin., Trump Administration Launches “Winning on Reducing Food Waste” Initiative (Oct. 18, 2018), https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm623790.htm [https://perma.cc/UZH3-5QF4] (“The [joint] agreement is aimed at improving coordination and communication across federal agencies attempting to better educate Americans on the impacts and importance of reducing food loss and waste.”). ↑
- U.S. Envtl. Prot. Agency, Winning on Reducing Food Waste Federal Interagency Strategy (April 9, 2019), https://www.epa.gov/sustainable-management-food/winning-reducing-food-waste-federal-interagency-strategy[https://perma.cc/8VND-TPSJ]. ↑
- 21 C.F.R. § 112.112 (2018). ↑
- Produce Safety Rule Final EIS, supra note 217, at 10-11, 10-12 (addressing food waste only in the context of “[s]oil [a]mendment,” which includes “table waste,” which includes all “post-consumer food waste”). The FDA did reject a more intrusive alternative that would have required farmers to ensure exclusion of animals from produce fields. Id. at 4-85 to 4-91 (discussing potential alternatives to rule regarding animal incursion to produce fields). The FDA’s “Waste Generation, Disposal, and Resource Use” focuses primarily on consequences of potential shifts from biological soil amendments such as compost and manure to chemical fertilizers, and does not directly consider wasted food. Id. at 4-7, 4-82, 4-89, 5-23 (examining waste specifically in the context of rules related to excluding animals from fields and not addressing food waste). ↑
- Standards for Growing, Harvesting, Packing, and Holding of Produce for Human Consumption, 80 Fed. Reg. 74,354, 74,479 (Nov. 27, 2015) (to be codified at 21 C.F.R. pt 11,16,112). ↑
- See Pollans, Regulating Farming, supra note 4, at 425. The Produce Safety Rule has staggered compliance dates for farms based on their size. The effective date for most farms was January 2018; for small farms, the effective date was January 2019, and for very small farms the effective date will be January 2020. U.S. Food & Drug Admin., Compliance Dates, https://www.fda.gov/Food/GuidanceRegulation/FSMA/ucm540944.htm [https://perma.cc/628K-L9VD]. ↑
- FSMA § 206, 21 U.S.C. § 350l (2012). ↑
- See, e.g., Scott Cameron Lougheed, Disposing of Risk: The Biopolitics of Recalled Food and the (Un)making of Waste, (Dec. 2017) (unpublished Ph.D. Dissertation, Queen’s University) (on file with author) (describing how recalls affect Canadian consumers and the Canadian food system). ↑
- Gunders, supra note 74, at 15. ↑
- Id. ↑
- See, e.g., Karen Grigsby Bates, Salmonella Scare Hurts Calif. Tomato Growers, NPR (July 9, 2008), https://www.npr.org/templates/transcript/transcript.php?storyId=92371196 [https://perma.cc/P2UF-S32A], (describing effects of salmonella scare for California tomato growers even though that region was not implicated in the outbreak). ↑
- 21 U.S.C. § 3501 (2012) (providing the FDA mandatory recall authority but making no mention as to methods of disposal for recalled food). ↑
- See U.S. Food & Drug Admin., Investigations Operation Manual, at Ch. 7 – Recall Activities (2018) (establishing procedure for ensuring recalled goods remain out of the stream of commerce, proper notice to distributors and consumers, and identification of the origin of contamination); Food Safety & Inspection Serv., Directive 8080.1 Rev. 7, Recall of Meat and Poultry Products (2013) (providing the “terminology, responsibilities, and public notification procedures regarding the voluntary recall of FSIS inspected meat and poultry products”); USDA, Guidelines for the Disposal of Intentionally Adulterated Food Products and the Decontamination of Food Processing Facilities 23–26 (2006) (providing guidelines for disposal by landfill and disposal by combustion); USDA, How to Develop a Meat and Poultry Product Recall Plan 15 (2013) (providing guidance to industry on how to ensure control of products during recall processes and focusing on the importance of establishing the certainty of disposal). ↑
- U.S. Food & Drug Admin., Investigations Operation Manual, supra note 241, at Ch. 7 – Recall Activities, § 7.2.1(8) (2018) (“FDA must witness or otherwise verify the reconditioning or destruction of the products returned under the recall . . . .”). ↑
- Food Safety & Inspection Serv., Directive 8080.1 Rev. 7 Recall of Meat and Poultry Products, Attachment 3, Product Recall Guidelines for Firms, § 1(H) (2013) (“Agency personnel should be notified prior to disposition actions (e.g., destruction or relabeling) of product returned to the firm.”). ↑
- There is one exception to this general treatment of recalls. The FDA provides detailed guidance on the repurposing and reconditioning of goods after disasters, including floods, earthquakes, hurricanes, volcanoes, tornadoes, chemical spills, wrecks, riots and disorders, fires, explosions, and bioterrorism. See U.S. Food & Drug Admin., Investigations Operation Manual, supra note 241, at Ch. 8.5 – Disaster Procedures, § 8.5.1 (disaster types). Goods affected by a disaster may be released into the stream of commerce, condemned for destruction or disposal, or reconditioned for a non-food, non-feed industry. See id. §§ 8.5.7.6–7.8. ↑
- Emily Broad Leib et al., Consumer Perceptions of Date Labels: Nat’l Survey 1 (2016), https://www.chlpi.org//wp-content/uploads/2013/12/Consumer-Perceptions-on-Date-Labels_May-2016.pdf [https://perma.cc/3YCU-9GUH]; Emily Broad Leib et Al., The Dating Game: How Confusing Food Date Labels Lead to Food Waste in America 19 (Dana Gunders ed. 2013) [hereinafter Broad Leib et al., The Dating Game]. ↑
- Broad Leib et al., The Dating Game, supra note 245, at 22. ↑
- Id. at 12 (“Because federal regulation of date labels is so limited, states consequently have vast discretion to regulate date labels in almost any way they see fit . . . The result is an inconsistent state regulatory scheme that is not necessarily improving public health.”). ↑
- Id. at 22 (“Laws in 20 states plus the District of Columbia also explicitly regulate the sale (and sometimes even donation) of foods beyond their label date.”). Montana, for example, prohibits sale or donation of past-date milk. Id. at 46 (citing Mont. Admin. R. 32.8.202(1) (2013) (“When 12 days or more have passed following pasteurization of a unit of grade A milk, there will be no quantities of that unit of milk sold or otherwise offered for public consumption.”)). ↑
- ReFED, supra note 220, at 20, 33. ↑
- See 21 U.S.C. § 331(a) (2012) (prohibiting introduction to interstate commerce of misbranded foods); id. § 371 (authorizing FDA enforcement); id. § 321(k) (defining “label”). ↑
- The FDA does note that sell by, use by, and best by dates “are quality dates only, not safety dates. If stored properly, a food product should be safe, wholesome and of good quality after its Use by or Best by date.” U.S. Food & Drug Admin., How to Cut Food Waste and Maintain Food Safety 2 (2018), https://www.fda.gov/downloads/Food/ResourcesForYou/Consumers/UCM529509.pdf [https://perma.cc/2HM6-AVZ4]. ↑
- Open Letter from Frank Yiannas, Deputy Commissioner, Food Policy and Response, Food & Drug Admin. (May 23, 2019), https://www.fda.gov/media/125114/download [https://perma.cc/PWU4-TPYW]. ↑
- See Broad Leib et al., The Dating Game, supra note 245, at 19 (citing Mary Bender Brandt et al., Ctr. for Food Safety & Applied Nutrition, Prevalence of Food Safety, Quality, and Other Consumer Statements on Labels of Processed, Packaged Foods, 23 Food Protection Trends 870, 872 (2003)) (noting that the FDA’s Center for Food Safety and Applied Nutrition has found most foods “when kept in optimal storage conditions, are safe to eat and of acceptable quality for periods of time past the label date”). ↑
- News Release, USDA Revises Guidance on Date Labeling to Reduce Food Waste, Food Safety & Inspection Serv. (Dec. 14, 2016), https://www.fsis.usda.gov/wps/portal/fsis/newsroom/news-releases-statements-and-transcripts/news-release-archives-by-year/archive/2016/nr-121416-01 [https://perma.cc/W7FP-SAJF]. ↑
- Emily M. Broad Leib, et al., Date Labels: The Case for Federal Legislation 6 (2019), https://www.chlpi.org/wp-content/uploads/2013/12/date-labels-issue-brief_June-2019.pdf [https://perma.cc/U6D5-5R3W]. ↑
- See Office of Tech. Assessment, Open Shelf-Life Dating of Food 21 (1979), www.princeton.edu/~ota/disk3/1979/7911/7911.PDF [https://perma.cc/S8FL-JLGS]; Dan Charles, Don’t Fear That Expired Food, NPR (Dec. 26, 2012), https://www.npr.org/templates/transcript/transcript.php?storyId=167819082 [https://perma.cc/J8FA-XH8D] (John Ruff, President of the Institute of Food Technologists, stating “[i]n forty years in eight countries, if I think of major product recalls and food poisoning outbreaks, I actually can’t think of one that has been driven by a shelf-life issue.”). ↑
- The FDA distinguishes “[p]ackaging,” which describes “placing food into a container that directly contacts the food and that the consumer receives,” and “[p]acking,” which describes “placing food into a container other than packaging the food.” 21 C.F.R. § 1.227 (2018). In this Article, we use “packaging” in its more colloquial sense. We also consider other kinds of single use resources such as disposable wipes. ↑
- There are sixty-six state agencies with jurisdiction over restaurants and retail food stores. Out of these agencies, sixty-three (and at least one in every state) have adopted some version of the FDA Model Food Code. U.S. Food & Drug Admin., Adoption of the FDA Food Code by State and Territorial Agencies Responsible for the Oversight of Restaurants and Retail Food Stores, 5–6 (2016), https://www.fda.gov/downloads/Food/GuidanceRegulation/RetailFoodProtection/FoodCode/UCM577858.pdf [https://perma.cc/V85M-6Z7T]. ↑
- Food Code § 3-304.11 (U.S. Food & Drug Admin. 2017). ↑
- Id. § 3-304.14(B) (discussing “cloths in-use for wiping counters”) (cross-referencing to requirements for the sanitizing bath). Although the Food Code requires that single-use disposable wipes “be used in accordance with EPA-approved manufacturer’s label use instructions,” these instructions relate to safety of use and not to disposal. Id. § 3-304.14(F). ↑
- Id. § 3-301.11(B). ↑
- Id. §§ 2.301.12, .14, .16 (establishing extensive requirements for handwashing); id. § 3.301.11(E) (establishing extensive requirements for when food may touch with bare hands). ↑
- Id. § 3-304.16(A). ↑
- 21 C.F.R. § 112.116(b) (2018). ↑
- See Produce Safety Rule Final EIS, supra note 217, at 2-37 (excluding Subparts K, dealing with packing and holding of food, and L, dealing with equipment sanitation, from further consideration in the EIS). According to the FDA, “[n]umerous state health regulations require clean, safe, and pest-free environments in which food is handled,” and although these regulations “do not necessarily extend to farms and farm mixed-type facilities, there is ample industry guidance for growers.” Id. The FDA concluded that “these actions are not expected to result in significant environmental impacts.” Id. ↑
- The Be Straw Free Campaign, National Park Service, https://www.nps.gov/articles/straw-free.htm [https://perma.cc/HBB3-4KH5]. ↑
- Plastic-Free Eats: How Restaurant Owner/Operators Can Reduce Plastic in Food Preparation and Service, Plastic Pollution Coalition, http://www.plasticpollutioncoalition.org/plastic-free-eateries [https://perma.cc/KT58-TH2D]. About 40 billion plastic utensils are produced each year. Nancy Trent, Ending Take Out Waste, Whole Foods Mag. (Jan. 24, 2011), https://wholefoodsmagazine.com/blog/ending-take-out-waste/ [https://perma.cc/QVH4-RE6P]. ↑
- Shauna Dineen, The Throwaway Generation: 25 Billion Styrofoam Cups a Year, Envtl. Mag. 35 (2005). ↑
- Laurel Curran, Gloves Alone Aren’t Enough for Food Safety, Food Safety News (Oct. 11, 2010) (noting the potential that improper use of gloves may exacerbate microbial risks). ↑
- Kenneth Marsh & Betty Bugusu, Food Packaging—Roles, Materials, and Environmental Issues, 72(3) J. Food Sci. R39, R40–R43 (2007) (describing various materials used in food packaging). ↑
- Rochman et al., supra note 76; see supra Part I.A.3 (describing these concerns in greater depth). ↑
- U.S. Envtl. Prot. Agency, Reducing Wasted Food & Packaging: A Guide for Food Services and Restaurants 3, https://www.epa.gov/sites/production/files/2015-08/documents/reducing_wasted_food_pkg_tool.pdf [https://perma.cc/T6SS-3BV5] (putting source reduction first in the food recovery and packaging waste prevention hierarchy). By contrast, some states and municipalities have taken steps to eliminate certain single use items. Plastic bags and Styrofoam are common targets of these laws. See, e.g., Cal. Pub. Res. Code § 42283–84 (2016) (prohibiting stores from “provid[ing] a single-use carryout bag to a customer”). ↑
- See Food Poisoning, Mayo Clinic (July 15, 2017), http://www.mayoclinic.org/diseases-conditions/food-poisoning/symptoms-causes/dxc-20337613 [https://perma.cc/ZV8H-YU34]. ↑
- U.S. Dept. of Agric. & U.S. Food & Drug Admin., Food Safety for People with Diabetes: A Need-to-Know Guide for Those Who Have Been Diagnosed with Diabetes 3 (2011), https://www.fda.gov/food/foodborneillnesscontaminants/peopleatrisk/ucm312706.htm [https://perma.cc/6NTT-E6P7]. ↑
- Id. at 3. ↑
- Barbara M. Lund & Sarah J. O’Brien, The Occurrence and Prevention of Foodborne Disease in Vulnerable People, 8 Foodborne Pathogens & Disease 961, 966 (2011). ↑
- Mayo Clinic, supra note 273. ↑
- U.S. Dept. of Agric. & U.S. Food & Drug Admin., Food Safety for People with Cancer: A Need-to-Know Guide for Those Who have Been Diagnosed with Cancer, 3 (2011), https://www.fda.gov/food/foodborneillnesscontaminants/peopleatrisk/ucm312565.htm [https://perma.cc/7VX8-TR5Y]. ↑
- See Ctrs. for Disease Control & Prevention, National Diabetes Statistics Report, supra note 154, at 10; Nat’l Ctr. for Health Statistics, Health, United States, 2016: with Chartbook on Long-term Trends in Health 221, at tbl.53 (2016), https://www.cdc.gov/nchs/data/hus/hus16.pdf#053 [https://perma.cc/9DUG-VPS8]; Nat’l Heart, Lung, & Blood Inst., Managing Overweight & Obesity in Adults: Systematic Evidence Review From the Obesity Expert Panel, 2013, at 3 (2013), https://www.nhlbi.nih.gov/sites/default/files/media/docs/obesity-evidence-review.pdf [https://perma.cc/7AJV-98K8] (explaining that obesity increases the “risk for morbidity from . . . type 2 diabetes . . . and some cancers”). ↑
- See Pollans, Regulating Farming, supra note 4, at 431–32, 431 n.141 (noting that requiring employees to wash their hands before handling food is one of the most effective known food safety measures). ↑
- 21 C.F.R. § 117.10(a) (2018) (“Any person who, by medical examination or supervisory observation, is shown to have, or appears to have, an illness, open lesion, including boils, sores, or infected wounds, or any other abnormal source of microbial contamination by which there is a reasonable possibility of food, food-contact surfaces, or food-packaging materials becoming contaminated, must be excluded from any operations which may be expected to result in such contamination until the condition is corrected, unless conditions such as open lesions, boils, and infected wounds are adequately covered.”). Although, as we discuss in Part II.C.3, such restrictions, when mandated in the absence of worker sick leave programs, might be counterproductive. ↑
- See Pollans, Regulating Farming, supra note 4, at 400 (identifying this as a major source of FSMA criticism). ↑
- Carrie Hribar, Understanding Concentrated Animal Feeding Operations and Their Impact on Communities 8–10 (2010), https://www.cdc.gov/nceh/ehs/docs/understanding_cafos_nalboh.pdf [https://perma.cc/WP25-FVGN] (describing pathogenic microorganisms linked to CAFOs). ↑
- For many CAFOs, the question is not if but when a spill will occur and how bad it will be. Although there is not accurate national level data, examples from a number of states and regions illustrate this point. For instance, a 1995 study in North Carolina found that about 55 percent of manure lagoons on hog farms were leaking. Waste Management, Sustainable Table, http://www.sustainabletable.org/906 [https://perma.cc/XKF2-KKMQ] (citing R.L. Huffman & P.W. Westerman, Estimated Seepage Losses From Established Swine Waste Lagoons in the Lower Coastal Plain in North Carolina, 38 Transactions of the Am. Soc’y of Agric. Eng’rs 449 (1995)). “In Indiana there are approximately 550 operating CAFOs. In 1997 animal feedlots were responsible for 2,391 manure spills in the state, including a single spill of 9,600 gallons of hog manure.” Indiana Must Require Industrial Farms to Have CWA Permits, or Face Loss of Its Authority: Save the Valley v. EPA, 23 Andrews Hazardous Waste Litig. Rep. 8 (2002). ↑
- See Multistate Outbreak of E. Coli O157:H7 Infections Linked to Romaine Lettuce (Final Update), Ctrs. for Disease Control & Prevention (June 28, 2018), https://www.cdc.gov/ecoli/2018/o157h7-04-18/index.html [https://perma.cc/995J-9AJ5]. ↑
- Environmental Assessment of Factors Potentially Contributing to the Contamination of Romaine Lettuce Implicated in a Multi-State Outbreak of E. coli O157:H7, U.S. Food & Drug Admin. (Nov. 1, 2018), https://www.fda.gov/Food/RecallsOutbreaksEmergencies/Outbreaks/ucm624546.htm [https://perma.cc/T2LG-A2X5]. ↑
- Id. (“FDA considers that the most likely way romaine lettuce became contaminated was from the use of water from this irrigation canal . . . A large concentrated animal feeding operation (CAFO) is located adjacent to this stretch of the irrigation canal. The EA team did not identify an obvious route for contamination of the irrigation canal from this facility; in addition, the limited number of samples collected at the CAFO also did not yield the outbreak strain. Other possible explanations for how the irrigation canal became contaminated are possible, but the EA team found no evidence in support of alternative explanations.”). ↑
- The FDA can regulate use of animal drugs on feedlots, but has no other direct regulatory authority. 21 U.S.C. § 360b (2012) (governing new animal drugs). Several other agencies, including the USDA and EPA, have authority over animal feedlots. See 40 C.F.R. § 122.21 (2018) (governing water discharge permits for CAFOs). ↑
- Standards for Growing, Harvesting, Packing, and Holding of Produce for Human Consumption, 80 Fed. Reg. 74,354, 74,456 (Nov. 27, 2015) (to be codified at 21 C.F.R. § pt 11, 16, 112) (describing what a farmer would need to do if he or she learned that a CAFO had opened upstream); see also Daniel S. Karp et al., Comanaging Fresh Produce for Nature Conservation and Food Safety, 112 Proceedings of the Nat’l Acad. of Scis. 11126, 11127 (2015) (finding that E. coli is about “100 times more likely” to be found near grazeable land than far from grazeable land); Pollans, Regulating Farming, supra note 4, at 439 (observing that the need to take steps to exclude wildlife from the farm may be more important at farms in the vicinity of CAFOs than those not). ↑
- 21 C.F.R. § 112.43(a)(1) (2018). ↑
- Standards for Growing, Harvesting, Packing, and Holding of Produce for Human Consumption, 80 Fed. Reg. 74,354, 74,551–52 (Nov. 27, 2015) (to be codified at 21 C.F.R. pt. 11,16,112); See also 21 C.F.R. §§ 112.11, .123(d)(1) (2018); 21 C.F.R. §§ 117.35, .37, .40 (2018); Final Human Preventive Control Rule, 80 Fed. Reg. 55,908, 55,956 (Sept. 17, 2015). ↑
- See Karp et. al, supra note 289, at 11126 (exploring the potential for comanaging farm environments to simultaneously “achieve food safety and nature conservation goals”). ↑
- Produce Safety Rule Final EIS, supra note 217, at 1-19 n.13. ↑
- Karp et al., supra note 289, at 11128. ↑
- Matthew S. Jones et al., Organic Farming Promotes Biotic Resistance to Foodborne Human Pathogens, 56 J. Applied Ecol. 1117, 1117 (2019) (concluding that “farmland simplification actually increases the likelihood that produce will be contaminated with human pathogens”). ↑
- Karp et al., supra note 289, at 11128. ↑
- Id.; Wild Farm All. & Cmty. All. with Family Farmers, A Farmer’s Guide to Food Safety and Conservation: Facts, Tips & Frequently Asked Questions (2d ed. 2017), https://d3n8a8pro7vhmx.cloudfront.net/wildfarmalliance/pages/131/attachments/original/1508942672/WFA_CAFF_FG_Farmer’s_Guide_to_Food_Safety_and_Conservation_10.23.17.small.file.pdf?1508942672 [https://perma.cc/KT27-N4TN]. ↑
- See Pollans, Regulating Farming, supra note 4, at 423. ↑
- 21 C.F.R. § 112.83(b)(2) (2018); Standards for Growing, Harvesting, Packing, and Holding of Produce for Human Consumption, 80 Fed. Reg. 74,354, 74,485 (Nov. 27, 2015); Baur, Driscoll, & Karp, supra note 219, at 143–44 (describing the pressures that farmers experience to remove vegetated buffers and adopt other food safety measures). ↑
- Produce Safety Rule Final EIS, supra note 217, at 1-19. ↑
- See 80 Fed. Reg. at 74,365. In response to public comments requesting conservation requirements, the FDA indicated that it “continue[s] to encourage the co-management of food safety, conservation, and environmental protection . . . However, the commenters identified no reason that it would be necessary for FDA to go beyond the statements [it has] included in § 112.84 and create affirmative conservation-related requirements in this rule.” Id. ↑
- See NRDC v. FDA, 760 F. 3d 151, 153 (2d Cir. 2014) (“[R]esearch shows that bacteria that develop resistance to antibiotics used in animal feed can transfer to human beings and pose a risk to human health. For that reason, various public-interest organizations have sought to force the [FDA] to prohibit the use of certain antibiotics in animal feed.”); Emilie Aguirre, An International Model for Antibiotics Regulation, 72 Food & Drug L.J. 295, 298 (2017) (examining opportunities to apply the “democratic experimentalist” model internationally to reduce antibiotic use on food-producing animals as a way to address antibiotic resistance) [hereinafter Aguirre, An International Model]; Emilie Aguirre, Contagion Without Relief: Democratic Experimentalism and Regulating the Use of Antibiotics in Food-Producing Animals, 64 UCLA L. Rev. 550, 557 (2017) (“As the only state action in this critical area, ensuring the experimentalist implementation of the California law and securing its fate against preemption are crucial to addressing the threat that overuse of antibiotics poses to public health.”); Jonathan Anomaly, Ethics, Antibiotics, and Public Policy, 15 Geo. J.L. & Pub. Pol’y 999, 1006–08 (2017) (examining the moral tradeoffs in antibiotics regulation); Lisa Heinzerling, Undue Process at the FDA: Antibiotics, Animal Feed, and Agency Intransigence, 37 Vt. L. Rev. 1007, 1008–09 (2013) (arguing that FDA acted incorrectly by not withdrawing animal drug approvals for antibiotics used in livestock); Timothy F. Landers et al., A Review of Antibiotic Use in Food Animals: Perspective, Policy, and Potential, 127 Pub. Health Rep. 4, 6 (2012) (noting a long history of evidence finding a link between antibiotics given to food-producing animals and antibiotic resistance in humans); Susan A. Schneider, Beyond the Food We Eat: Animal Drugs in Livestock Production, 25 Duke Envtl. L. & Pol’y F. 227, 229 (2015) (arguing that the existing regulatory framework is insufficient); Sidney A. Shapiro, Overuse of Antibiotics in Concentrated Animal Feeding Operations: Regulation and Tort Law, 47 Envtl. L. 557, 558 (2017) (exploring the use of the tort system to regulate overuse of antibiotics). ↑
- See industrial food Animal Production in America: Examining the Impact of the Pew Commission’s Priority Recommendations, Johns Hopkins ctr. for a livable future 2 (2013), http://www.jhsph.edu/research/centers-and-institutes/johns-hopkins-center-for-a-livable-future/_pdf/research/clf_reports/CLF-PEW-for%20Web.pdf [https://perma.cc/7K9K-E7SW] (“Based on FDA data, 29.9 million pounds of antibiotics were sold for use in meat and poultry production in 2011, representing 80 percent of the total volume of antibiotics sold in the United States for any purpose.”). ↑
- See Landers et al., supra note 302, at 6 (describing the differences between “therapeutic use” (to treat sick animals), “prophylactic use” (to prevent disease), and “subtherapeutic use” (to promote growth)). ↑
- NRDC, 760 F.3d at 153 n.5 (“‘Subtherapeutic’ uses are those that seek ‘increased rate of [weight] gain, disease prevention[,] etc.,’ as opposed to uses to treat illnesses or other pathological conditions. Other sources prefer the term ‘nontherapeutic,’ for the same meaning.”) (citation omitted). ↑
- 21 U.S.C. § 360b (2012). Within the FDA, the Center for Veterinary Medicine (CVM) regulates animal drugs. About the Center for Veterinary Medicine (CVM), U.S. Food & Drug Admin., https://www.fda.gov/AboutFDA/CentersOffices/OfficeofFoods/CVM/default.htm [https://perma.cc/3J55-HHSF]. ↑
- 21 U.S.C. § 360b(e)(1)(A) (2012). ↑
- See NRDC, 760 F.3d at 180 (“The FDA argues that the formal withdrawal process contemplated by the statute can be expensive and time-consuming, and that its voluntary compliance strategy will reach the same result more quickly and at lower cost.”). ↑
- EU Commission Regulation 1831/2003 of Sept. 22, 2003, On Additives for Use in Animal Nutrition, art. 11, 2003 O.J. (L 268) 29, 36 (EC) (banning the use of antibiotics for growth promotion purposes, but still allowing their use for disease prevention); Carol Cogliani et al., Restricting Antimicrobial Use in Food Animals: Lessons from Europe, 6 Microbe 274, 274 (2011) (describing bans on antibiotic use for food animal growth promotion in Denmark, Sweden, and the United Kingdom). ↑
- Aguirre, An International Model, supra note 302, at 297 (“Forty years after threatening to withdraw approval for subtherapeutic use of antibiotics, the U.S. Food and Drug Administration . . . has failed to follow through, instead promulgating a set of voluntary guidelines for industry to follow.”); Heinzerling, supra note 302, at 1011 (describing how, in 1977, the FDA proposed to withdraw approval for penicillin and tetracycline use in food animals, but that it eventually withdrew its hearing notice and stated that “voluntary measures by the animal feed industry were a better idea”). ↑
- See Guidance for Industry #209: The Judicious Use of Medically Important Antimicrobial Drugs in Food-Producing Animals, U.S. Food & Drug Admin. Ctr. for Veterinary Med. 21–22 (2012), https://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM216936.pdf [https://perma.cc/3UHE-H7TC] (providing a voluntary framework to reduce the use of medically-important antibiotics in animal production by focusing on two principles: (1) limiting use of such drugs to those that are needed for animal health, and (2) limiting use of such drugs to instances with veterinary oversight); Guidance for Industry #213: New Animal Drugs and New Animal Drug Combination Products Administered In or On Medicated Feed or Drinking Water of Food-Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with GFI #209, U.S. Food & Drug Admin. Ctr. for Veterinary Med. 10–17 (2013), https://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM299624.pdf [https://perma.cc/ZC6P-ACGG] (explaining how new animal drug sponsors can voluntarily comply with the principles outlined in GFI #209). ↑
- See Guidance for Industry #209, supra note 311, at 21. ↑
- See Antimicrobial Animal Drug Sales and Distribution Reporting, 80 Fed. Reg. 28,863 (May 20, 2015) (to be codified at 21 C.F.R. § 514.87). ↑
- Antibiotic Resistance Threats in the United States, 2013, Ctr. for Disease Control 6 (2013), http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf [https://perma.cc/2LLT-SZCD]. ↑
- Id. at 11. ↑
- See id. at 37. ↑
- Standards for Growing, Harvesting, Packing, and Holding of Produce for Human Consumption, 80 Fed. Reg. 74,354, 74,375 (Nov. 27, 2015) (to be codified at 21 C.F.R. pt 11,16,112) (identifying worker hygiene as a leading pathway of contamination); see generally Employee Health and Personal Hygiene Handbook, U.S. Food & Drug Admin., http://www.fda.gov/Food/GuidanceRegulation/RetailFoodProtection/IndustryandRegulatoryAssistanceandTrainingResources/ucm113827.htm [https://perma.cc/4XG7-E8E5]. ↑
- See, e.g., 21 C.F.R. § 117.10(a) (2016) (requiring that anyone who appears to be sick “be excluded from any operations which may be expected to result in [microbial] contamination”). ↑
- See Food Code § 2-201.11(a)(5) (U.S. Food & Drug Admin. 2017). ↑
- See id. § 2-201.12 (requiring employees be restricted from the work area who demonstrate a range of symptoms or have a variety of diagnoses). ↑
- Hidden Tragedy: Underreporting of Workplace Injuries and Illnesses: Hearing Before the H. Comm. on Educ. and Labor U.S. House of Representatives, 110th Cong. 18–19 (2008) (statement of John Rusher, Assistant Commissioner for Safety and Health Statistics, Bureau of Labor Statistics) (exploring why both injuries and illnesses are often underreported); see also id. at 12 (statement of A.C. Span, Former Employee for Basha Distribution Center) (describing a work environment’s atmosphere and what led those employees to underreport). ↑
- The Food Code requires that an excluded worker be reinstated once the employee has been asymptomatic for 24–48 hours, depending on the condition, and, in some cases, a doctor’s sign off is also required. See Food Code § 2-201.13. ↑
- Food safety practices can generate other kinds of economic justice concerns as well. Patrick Baur, Christy Getz & Jennifer Sowerwine, Contradictions, Consequences and the Human Toll of Food Safety Culture, 34 Agric. & Hum. Values 713 (2017) (describing the consequences of “the bureaucratization of food safety power on the everyday routines and lived experiences of people working to grow, pack, and deliver fresh produce”). ↑
- Diana Stuart & Michelle R. Worosz, Risk, Anti-Reflexivity, and Ethical Neutralization in Industrial Food Processing, 29 Agric. & Hum. Values 287, 287–88 (2012). ↑
- See id. ↑
- See id. at 291, 293. ↑
- Multistate Outbreak of Listeriosis Linked to Whole Cantaloupes from Jensen Farms, Colorado, Ctrs. for Disease Control & Prevention (Aug. 27, 2012), https://www.cdc.gov/listeria/outbreaks/cantaloupes-jensen-farms/index.html [https://perma.cc/A2BS-EZYE]. ↑
- Id. ↑
- Multistate Outbreak of E. coli O157:H7 Infections Linked to Fresh Spinach, Ctrs. for Disease Control & Prevention (Oct. 6, 2006), https://www.cdc.gov/ecoli/2006/spinach-10-2006.html [https://perma.cc/9X3Q-PB4V]. ↑
- See FSMA § 204, 21 U.S.C. § 2223 (2012) (establishing procedures for “[e]nhancing tracking and tracing of food and recordkeeping”). ↑
- See John Bovay, et al., Estimated Costs for Fruit and Vegetable Producers to Comply with the Food Safety Modernization Act’s Produce Rule, U.S. Dept. of Agric., Econ. Research Serv. 19 tbl.7 (2018), https://www.ers.usda.gov/webdocs/publications/89749/eib-195.pdf?v=0 [https://perma.cc/2TJP-RFA6] (assessing compliance costs as a share of revenue by farm size). ↑
- Id.; Produce Safety Final RIA, supra note 205, at 101–02 tbl.34 (FDA’s estimated per-farm compliance costs were $5,872 for “very small” farms and $24,683 for “small” farms.). ↑
- See 21 U.S.C. § 350h(f). This amendment exempts “small” farms (farms with less than $500,000 of annual revenue) that receive more than 50 percent of their revenue from sales to qualified end-users (defined as consumers, restaurants, or retailers within the same states as the farm or within 275 miles). See id. ↑
- 21 U.S.C. § 350h(a)(1)(B); 21 C.F.R. § 112.4 (2017) (providing an exemption for farms that sold less than $25,000 in produce per year). ↑
- See supra notes 129–139 (describing the influence of private governance and buyer preference on farmer behavior). ↑
- See Broad Leib, supra note 212, at 47. ↑
- See Where’s the Local Beef? Rebuilding Small-Scale Meat Processing Infrastructure, Food & Water Watch (2009), https://www.foodandwaterwatch.org/sites/default/files/wheres_the_local_beef_report_june_2009_0.pdf [https://perma.cc/8V5F-DMU8]; Luis A. Ribera & Ronald D. Knutson, The FDA’s Food Safety Modernization Act and Its Economic Implications, 26 Choices 4 (2011), http://www.choicesmagazine.org/UserFiles/file/cmsarticle_197.pdf [https://perma.cc/46N5-4FKU] (describing how HACCP’s high compliance costs hurt smaller meat plants more than larger plants). ↑
- Weiss, supra note 8, at 98 (explaining that a problem definition can “invite[] participation by some political actors and devalue[] the involvement of others”). ↑
- See, e.g., Renée A. Irvin & John Stansbury, Citizen Participation in Decision Making: Is It Worth the Effort?, 64 Pub. Admin. Rev. 55, 56 (2004) (listing potential advantages of broad participation). ↑
- See Pollans, FDA and the Environment, supra note 91. ↑
- Consumers interested in using information to choose among food products on the basis of personal health, environmental impact, supply chain equity, or animal welfare face a number of barriers. First, consumers are often inundated with too much information that they do not have the tools to sort through. Second, the regulatory landscape around food labeling is complicated and can generate confusion. See, e.g., Silverglade & Heller, supra note 60, at VI-1 (describing the “chaos” of food labels). Finally, current methods for measuring impacts in each of these areas are limited, so measures that are available can be misleading. Czarnezki, Pollans & Main, supra note 86, at 1008–21 (listing shortcomings of current impact valuation methods and considering normative limits to use of eco-labeling to achieve food system change). ↑
- The GAO’s mission is to support Congress and to improve the performance and accountability of the federal government. Draft: GAO’s Strategic Plan for Serving the Congress 2004–2009, U.S. Gen. Acct. Off. 3–5 (2003), https://www.gao.gov/dsp/SPDraft.pdf [https://perma.cc/VXV7-X8C3]. Among other things, it evaluates the effectiveness of federal programs, conducts audits of federal spending, and offers analyses of proposed actions. Id. GAO reports can shed light on government malfeasance and can spur congressional action. Id. The USDA’s Economic Research Service conducts economic research on “agriculture, food, the environment, and rural America” to support public and private decision making. About ERS, U.S. Dept. of Agric., https://www.ers.usda.gov/about-ers/ [https://perma.cc/X472-EK8V]. ↑
- We might, for instance, rename the primary problem with a phrase we have used a number of times throughout this Article: food system health risks. This name is descriptively accurate and is useful in establishing the outer bounds of the problem, but it lacks the urgency and specificity of the phrase “food safety.” ↑
- Lecos, supra note 11, at 388. ↑
- Jack Ringquist et al., Capitalizing on the Shifting Consumer Food Value Equation, Deloitte 18 (2016), https://www2.deloitte.com/content/dam/Deloitte/us/Documents/consumer-business/us-fmi-gma-report.pdf [https://perma.cc/WMM2-HWEN] (concluding that a “meaningful number of consumers now think about Safety from a holistic, longer-term perspective”). ↑
- Id. at 17 & 17 fig.17. The survey question asked consumers to select which of the following criteria they believe are the top five attributes of safety: “free of harmful elements” (62% said yes); “clear and accurate labeling” (51%); “clear information” (47%); “fewer ingredients” (42%); and “nutritional content” (41%). Id. Although fewer consumers selected nutritional content than any of the other categories, the other four are tied more directly to traditional food safety. ↑
- Id. at 18 & 18 fig.18. The survey itself characterized “environmental responsibility” and “fair treatment of workers” as “social impact” issues. Id. 48 percent of consumers selected “fair treatment of workers” as a reason for selecting a retailer, and 39 percent selected “environmental responsibility.” Id. ↑
- 81 percent of consumers surveyed were willing to pay more for healthier foods, but only 5 percent of consumers surveyed identified social impact as a “driver” in purchasing decisions. Id. at 16, 18, 19. Another study found that 79 percent of consumers “are willing to pay more . . . for fruits and vegetables produced by workers who earned a living wage and were treated fairly.” Food Labels Survey: 2016 Nationally-Representative Phone Survey, Consumer Rep. Nat’l. Res. Ctr. 6 (2016), https://www.ftc.gov/system/files/documents/public_events/975753/cr_intro_and_2016_food_survey.pdf [https://perma.cc/TM3L-MHFV]. ↑
- See, e.g., C. Francis et al., Agroecology: The Ecology of Food Systems, 22 J. Sustainable Agric. 99, 99 (2003) (arguing that that definition of agroecology should encompass ecological, economic, and social perspectives); see also Nicholas Freudenberg et al., Can a Food Justice Movement Improve Nutrition and Health? A Case Study of the Emerging Food Movement in New York City, 88 J. Urban Health 623, 629 (2011) (grouping environmental, equity, and health issues). Even mainstream institutions such as the National Institute of Medicine and the National Research Council have embraced this approach. See Malden C. Nesheim et al., Institute of Medicine & National Research Council, A Framework for Assessing Effects of the Food System 89 (2015) (“Food system interventions are more likely to succeed if they are informed by an understanding of the intrinsic dynamics associated with public health, environmental, and social and economic outcomes, and an appreciation that their interactions are nonlinear and not always readily predicted.”). ↑
- Toward a Healthy Sustainable Food System, Am. Pub. Health Ass’n (2007), https://www.apha.org/policies-and-advocacy/public-health-policy-statements/policy-database/2014/07/29/12/34/toward-a-healthy-sustainable-food-system [https://perma.cc/XW6N-G6XF]. ↑
- U.S. Gov’t. Accountability Office, GAO-15-38, Food Safety: FDA and USDA Should Strengthen Pesticide Residue Monitoring Programs and Further Disclose Monitoring Limitations 55–57 (2014), https://www.gao.gov/assets/670/666408.pdf [https://perma.cc/58JV-87M5] (calling on FDA and USDA to improve pesticide monitoring programs); Office of Inspector Gen., OEI 02-14-00420, Challenges Remain in FDA’s Inspections of Domestic Food Facilities 9–10, 13 (2017), https://oig.hhs.gov/oei/reports/oei-02-14-00420.pdf [https://perma.cc/S46Q-CRVJ] (identifying challenges in meeting inspection targets and finding that over 20 percent of the time FDA takes no action after uncovering significant inspection violations); Silverglade & Heller, supra note 60, at Part XI-1, XI-3, XI-5 (critiquing FDA for underenforcement on a variety of issues). ↑
- None of the paths laid out in this Section include a strategy for overcoming the political, cultural, and economic barriers to more robust health regulation that we identified in Part I.B. Instead, we optimistically rely on three trends to help facilitate these changes: growing consumer interest in nutrition and social impacts of food, increasing awareness among policymakers about the interconnections of the food system, and heightening urgency related to climate change and to rising health care costs, for food system change. ↑
- In fact, we recommend that Congress not grant the FDA full authority over broad food safety. The FDA’s expertise lies in ingestion-related harm. Some limited authority over environmental and workplace safety impacts might be appropriate, see Pollans, FDA and the Environment, supra note 91, but FDA is not well-situated to implement a comprehensive mandate in this area. ↑
- 21 U.S.C. § 321(u) (2012). This definition applies to food additives (§ 321(s) and § 348), new animal drugs (§ 360b and § 360c), and color additives (§ 379e). ↑
- As discussed in Part II.A, the FDA currently allocates the vast majority of its food safety and nutrition-related budget to food safety, and, while it articulates numerous nutrition goals, it does not dedicate significant financial resources to them. ↑
- See U.S. Gov’t Accountability Office, GAO-18-174, supra note 170. ↑
- See supra note 351 (citing several reports critiquing FDA’s enforcement record). In addition, after Congress passed FSMA, there was widespread concern that the agency had insufficient financial resources to implement it. See, e.g., Ron Nixon, Funding Gap Hinders Law for Ensuring Food Safety, N.Y. Times (Apr. 7, 2015), https://www.nytimes.com/2015/04/08/us/food-safety-laws-funding-is-far-below-estimated-requirement.html [https://perma.cc/P6ZK-8N8L] (noting that for 2011–2015 Congress had appropriated less than half the amount that the Congressional Budget Office estimated the agency would need to implement the statute). Despite a significant increase to the agency’s budget in 2016, concerns remain about adequate funding. See Michael Taylor, Unfinished Business: Keeping the Focus on Food Safety, Food Safety News (July 9, 2018), https://www.foodsafetynews.com/2018/07/unfinished-business-keeping-the-focus-on-food-safety/ [https://perma.cc/3DH7-5BQA] (critiquing the Trump administration for not seeking to increase funding for FSMA implementation); Joseph James Whitworth, Congress Approves Increased Food Safety Funding, Food Navigator (Jan. 5, 2016), https://www.foodnavigator.com/Article/2016/01/05/FDA-food-safety-activities-get-funding-boost# [https://perma.cc/83JJ-XHYM] (describing funding increase of over $100 million). ↑
- See Freeman & Rossi, supra note 10, at 1151 (explaining the benefits of “shared regulatory space”). ↑
- Id. at 1155, 1157, 1173, 1176. ↑
- Emily Broad Leib et al., Blueprint for a National Food Strategy: Evaluating the Potential for a National Food Strategy in the United States 10 (2017) [hereinafter Broad Leib et al., Blueprint]. ↑
- Id. at 21. ↑
- See id. at 14. ↑
- HM Gov’t, Dep’t for Env’t, Food & Rural Affairs, Food 2030, at 4 (2010), http://nourisheu.com/wp-content/uploads/2015/02/food2030strategy.pdf [https://perma.cc/8DFV-D9AC]. ↑
- See id. The strategy identifies “six core issues for the food system”: (1) “[e]ncouraging people to eat a healthy, sustainable diet”; (2) “[e]nsuring a resilient, profitable and competitive food system”; (3) “[i]ncreasing food production sustainably”; (4) “[r]educing the food system’s greenhouse gas emissions”; (5) “[r]educing, reusing and reprocessing waste”; and (6) “[i]ncreasing the impact of skills, knowledge, research and technology.” Id. at 9. ↑
- See Broad Leib et al., Blueprint, supra note 360, at 46–47 (discussing dedicated offices and “czars” and interagency working groups and advisory councils). Examples of interagency working groups include the National Strategy for Combating Antibiotic-Resistant Bacteria’s Task Force, the National Quality Strategy’s Interagency Working Group on Healthcare Quality, and the Interagency Working Group on Environmental Justice. Id. at 38–39. ↑
- Id. at 47–48. ↑
- See U.S. Gov’t Accountability Office, GAO-15-290, High-Risk Series: An Update 264 (2015), http://www.gao.gov/assets/670/668415.pdf [https://perma.cc/5U8M-NB78]. ↑
- Id. at 264–65. ↑
- Id. at 265–66. The report found that FDA and FSIS have numerous coordination mechanisms, but they are issue specific, and “none provides for broad-based, centralized collaboration. For example, FDA and FSIS are collaborating with CDC through the Interagency Food Safety Analytics Collaboration to improve estimates of the most common sources of foodborne illnesses.” Id. The report concludes that “without a centralized collaborative mechanism on food safety—like the FSWG—there is no forum for agencies to reach agreement on a set of broad-based food safety goals and objectives that could be articulated in a government-wide performance plan on food safety.” Id. ↑
- Food Safety: A Unified, Risk-Based Food Safety System Needed: Testimony Before the Subcomm. on Human Res. & Intergovernmental Relations of the H. Comm. on Gov’t Operations 1 (1994) (statement of John W. Harman, Director, Food and Agriculture Issues, Resources, Community, and Economic Development Division), https://www.gao.gov/assets/110/105575.pdf [https://perma.cc/7A93-7YK2] (critiquing fragmentation in food safety and calling for creation of a single agency). ↑
- U.S. Gov’t Accountability Office, GAO-15-180, Federal Food Safety Oversight: Additional Actions Needed to Improve Planning and Coordination 2 (2014), https://www.gao.gov/assets/670/667656.pdf [https://perma.cc/NZ4M-GB7S] (recommending the creation of a government-wide food safety performance plan). ↑
- See generally, U.S. Gov’t Accountability Office, GAO-17-74, Food Safety A National Strategy is Needed to Address Fragmentation in Federal Oversight (2017), https://www.gao.gov/assets/690/682095.pdf [https://perma.cc/SD8B-ZMPQ] (calling for a national food safety strategy to address fragmentation). ↑
- Id. at 22. ↑
- See e.g., Broad Leib et al., Blueprint, supra note 360, at 10–12 (arguing that a national food strategy could engage relevant agencies and members of the public to set priorities and coordinate to address interrelated food system challenges including narrow food safety as well as obesity, food insecurity, food workers, and environmental degradation and natural resource usage); Advisory Committee on Biotechnology and 21st Century Agriculture Plenary Meeting, Summary 6 (Sept. 8–9, 2016), https://www.usda.gov/sites/default/files/documents/ac21-meeting-summary-september-2016.pdf [https://perma.cc/P824-U2TW] (noting that Secretary Vilsack “proposed that the [next] Administration consider establishing a Food Council”); Mark Bittman et al., How a National Food Policy Could Save Millions of American Lives, Wash. Post (Nov. 7, 2014), https://www.washingtonpost.com/opinions/how-a-national-food-policy-could-save-millions-of-american-lives/2014/11/07/89c55e16-637f-11e4-836c-83bc4f26eb67_story.html [https://perma.cc/6BVU-YR8C] (decrying the lack of a “food policy . . . for managing American agriculture or the food system as a whole” and calling for such a policy to address a range of issues, including providing healthy food access, eliminating “toxic bacteria, chemicals and drugs,” and implementing farm policies that “support our public health and environmental objectives”). ↑
- See Beyranevand & Broad Leib, supra note 4, at 239–40. ↑
- See Alejandro E. Camacho & Robert L. Glicksman, Reorganizing Government: A Functional and Dimensional Approach, Ch. 3 (NYU Press, forthcoming 2019) (draft at 5–8) (on file with authors) (providing a detailed history of proposals to consolidate traditional food safety governance and critiquing these proposals on the ground that they tend to treat consolidation as an all or nothing proposition and fail to differentiate by different functions such as research, inspection, and enforcement); infra note 383 (identifying some of these proposals). ↑
- Camacho & Glicksman, supra note 376 (draft at 2); Johnson, supra note 1, at 1. FDA and USDA dominate, but other agencies, such as the National Oceanic and Atmospheric Administration, Customs and Border Protection, Federal Trade Commission, and Alcohol and Tobacco Tax and Trade Bureau all play lesser roles in regulating aspects of the food system. See Johnson, supra note 1, at 2 (noting, for example, that the National Oceanic and Atmospheric Administration (NOAA) plays a food safety role in terms of “grading fish and seafood,” and that Customs and Border Protection (CBP) is responsible for food safety “front-line enforcement and referral”). ↑
- The EPA has exclusive authority over pesticide registration and approval. 7 U.S.C. § 136(a) (2012). It also sets pesticide residue levels. 21 U.S.C. § 346a(b)(1) (2012). FDA enforces pesticide residue levels and sets action levels for pesticides that have no established residue levels. Enforcement Activities on Misuse of Pesticides and Pesticide Contamination of Food, Notice of Agreement Between the Food and Drug Administration and U.S. Environmental Protection Agency, 40 Fed. Reg. 25,078 (June 12, 1975) (memorializing a Memorandum of Understanding between the two agencies); Hutt et al., supra note 7, at 634 (describing allocation of pesticide authority). ↑
- 21 U.S.C. § 360(b). ↑
- Meat and Poultry Labeling Terms, U.S. Dep’t of Agric., Food Safety & Inspection Serv., http://www.fsis.usda.gov/wps/portal/fsis/topics/food-safety-education/get-answers/food-safety-fact-sheets/food-labeling/meat-and-poultry-labeling-terms/meat-and-poultry-labeling-terms [https://perma.cc/ND3U-GNTG]. ↑
- See Lisa Heinzerling, Divide and Confound: The Relationship Between Transparency, Public Health, and Regulatory Authority in the National Food System, in Food and Drug Regulation in an Era of Globalized Markets 125, 126 (Sam Halabi ed., 2015) (discussing FDA and USDA’s authority over different types of frozen pizza). ↑
- U.S. Gov’t Accountability Office, GAO-17-317, High-Risk Series: Progress on Many High Risk Areas, While Substantial Efforts Needed on Others 389 (2017), http://www.gao.gov/assets/690/682765.pdf [https://perma.cc/BHE2-C4BF] (food safety has been on the list since 2007); High Risk List, U.S. Gov’t Accountability Office, http://www.gao.gov/highrisk/overview [https://perma.cc/2G2X-F2SN]. ↑
- See, e.g., Richard J. Durbin, Food Safety Oversight for the 21st Century: the Creation of a Single Independent Federal Food Safety Agency, 59 Food & Drug L.J. 383, 385 (2004) (concluding that “[a] single agency with authority based on science provides the greatest hope of reducing foodborne illnesses in this country”); Timothy M. Hammonds, It is Time to Designate a Single Food Safety Agency, 59 Food & Drug L.J. 427, 432 (2004) (noting that “the only way to carry out meaningful, long-lasting reform of the U.S. food regulatory system is to designate a true single agency with total regulatory authority for the safety of the entire food system”); Richard A. Merrill & Jeffrey K. Francer, Organizing Federal Food Safety Regulation, 31 Seton Hall L. Rev. 61, 67 (2000) (exploring “the obstacles that consolidation would face if undertaken seriously and discovering what past reorganization efforts suggest could be the effects of combining the existing programs in a single organization”); Michael R. Taylor, Lead or React? A Game Plan for Modernizing the Food Safety System in the United States, 59 Food & Drug L.J. 399, 401 (2004) (“When it comes to the safety and security of the food supply, and maintaining confidence among American consumers and trading partners, a single official and agency with both a clearly-defined food safety mission and accountability for success is needed.”). ↑
- See Safe Food Act of 2015, S. 287, 114th Cong. (2015). This bill was not taken up by any committee, and no similar legislation was re-introduced in the subsequent Congress. ↑
- Lydia Zuraw, Obama’s 2016 Budget: $1.6 Billion for Food Safety, Single Food-Safety Agency, Food Safety News (Feb. 3, 2015), http://www.foodsafetynews.com/2015/02/obama-2016-budget-includes-1-6-billion-for-food-safety/#.VVuHKRcmbW4 [https://perma.cc/Y4SF-HNWP]. ↑
- U.S. Exec. Office of the President, Delivering Government Solutions in the 21st Century: Reform Plan and Reorganization Recommendations 32–33 (2018), https://www.performance.gov/GovReform/Reform-and-Reorg-Plan-Final.pdf [https://perma.cc/47KD-R3J3]. ↑
- See Lily Rothman, Here’s Why the Environmental Protection Agency Was Created, Time (Mar. 22, 2017), http://time.com/4696104/environmental-protection-agency-1970-history/ [https://perma.cc/YN22-ESDK]. ↑
- Richard J. Lazarus, The Making of Environmental Law 69 (2004) (discussing rejection of a potential “Natural Resources and the Environment” agency). ↑
- See id. ↑
- Freeman & Rossi, supra note 10, at 1152 & 1152 n.84 (citing U.S. Dep’t of Homeland Sec., Brief Documentary History of the Department of Homeland Security: 2001–2008 (2008), https://www.hsdl.org/?view&did=37027 [https://perma.cc/5CHS-RCTS]); O’Connell, supra note 10. ↑
- See, e.g., Safe Food Act of 2015, S. 287, 114th Cong. (2015) (enumerating the functions that should be included in the single food safety agency, all of which fall within traditional food safety); Food Safety: U.S. Needs a Single Agency to Administer a Unified, Risk-Based Inspection System: Testimony Before the Subcomm. on Oversight of Gov’t Mgmt., Restructuring and D.C. of the S. Comm. On Gov’t Affairs 2 (1999), https://www.gao.gov/assets/110/108064.pdf [https://perma.cc/DYE9-6UBL] (describing the need for consolidation of food safety authority to address issues of foodborne illness like listeria in hot dogs and salmonella in eggs); Durbin, supra note 383, at 385 (supporting creation of a single food agency as “the greatest hope of reducing foodborne illnesses in this country”); Hammonds, supra note 383, at 428 (noting that a quintessential example of the need for a single agency is a food safety outbreak); Merrill & Francer, supra note 383, at 91–111 (describing all of the agency functions that would be combined into a single agency as the functions of FDA’s Center for Food Safety and Applied Nutrition, USDA’s Food Safety and Inspection Service, and several other agencies that are focused on acute poisoning from: microbial contamination, controlling potentially toxic chemicals in food, inspection of food and processing plants, and tracking of foodborne illness; all of these are part of traditional food safety); Taylor, supra note 383, at 399 (noting that “reform should focus the system more effectively on prevention of foodborne illness”). ↑
- See Biber, supra note 8, at 12 (explaining that agencies with multiple goals will often prioritize “easily measured goals” over competing goals that are difficult to measure). ↑
- See supra Parts I.A & I.B (describing some of these regulatory gaps). As we discuss above, the current allocation of regulatory responsibility is not simply an instance of rational division of labor. Cf. Todd S. Aagaard, Environmental Law Outside the Canon, 89 Ind. L.J. 1239, 1298 (2014) (arguing that environmental law should include more “dispersed, relatively small programs that . . . integrate environmental protection with other objectives”); Jacob E. Gersen, Overlapping and Underlapping Jurisdiction in Administrative Law, Sup. Ct. Rev. 201 (2006). ↑
- See Biber, supra note 8, at 35 (describing why, despite the challenges facing multiple-goal agencies, a single agency, even one with multiple goals, would be better than dividing up the goals into separate agencies because “the result would be higher transaction costs for the coordination efforts”). ↑
- Note, Reforming the Food Safety System: What If Consolidation Isn’t Enough?, 120 Harv. L. Rev. 1345 (2007); Diana R. H. Winters, Putting Humpty Dumpty Together Again: Consolidating Regulatory Authority Over Food Safety, Health Aff. Blog (Feb. 23 2015), http://m.healthaffairs.org/blog/2015/02/23/putting-humpty-dumpty-together-again-consolidating-regulatory-authority-over-food-safety/ [https://perma.cc/39GG-8LA2]. ↑
- U.S. Gen. Accounting Office, GAO/HRD-89-142, FDA Resources: Comprehensive Assessment of Staffing, Facilities, and Equipment Needed 5 (1989). No more recent estimate exists, but because governmental costs on the whole have risen, this number is likely higher today. ↑
- Freeman & Rossi, supra note 10, at 1152. ↑
- Id. at 1154. ↑
- Merrill & Francer, supra note 383, at 118–19 (discussing a 1977 report recommending consolidation of all food safety regulation under authority of the FDA). ↑